Downregulation of FoxC2 Increased Susceptibility to Experimental Colitis: Influence of Lymphatic Drainage Function?
- PMID: 25822012
- PMCID: PMC4437831
- DOI: 10.1097/MIB.0000000000000371
Downregulation of FoxC2 Increased Susceptibility to Experimental Colitis: Influence of Lymphatic Drainage Function?
Abstract
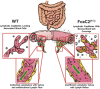
Background: Although inflammation-induced expansion of the intestinal lymphatic vasculature (lymphangiogenesis) is known to be a crucial event in limiting inflammatory processes, through clearance of interstitial fluid and immune cells, considerably less is known about the impact of an impaired lymphatic clearance function (as seen in inflammatory bowel diseases) on this cascade. We aimed to investigate whether the impaired intestinal lymphatic drainage function observed in FoxC2 mice would influence the course of disease in a model of experimental colitis.
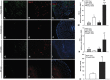
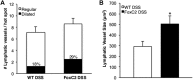
Methods: Acute dextran sodium sulfate colitis was induced in wild-type and haploinsufficient FoxC2 mice, and survival, disease activity, colonic histopathological injury, neutrophil, T-cell, and macrophage infiltration were evaluated. Functional and structural changes in the intestinal lymphatic vessel network were analyzed, including submucosal edema, vessel morphology, and lymphatic vessel density.
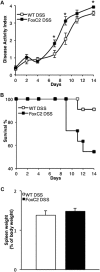
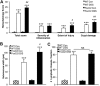
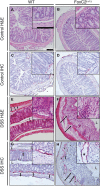
Results: We found that FoxC2 downregulation in FoxC2 mice significantly increased the severity and susceptibility to experimental colitis, as displayed by lower survival rates, increased disease activity, greater histopathological injury, and elevated colonic neutrophil, T-cell, and macrophage infiltration. These findings were accompanied by structural (dilated torturous lymphatic vessels) and functional (greater submucosal edema, higher immune cell burden) changes in the intestinal lymphatic vasculature.
Conclusions: These results indicate that sufficient lymphatic clearance plays a crucial role in limiting the initiation and perpetuation of experimental colitis and those disturbances in the integrity of the intestinal lymphatic vessel network could intensify intestinal inflammation. Future therapies might be able to exploit these processes to restore and maintain adequate lymphatic clearance function in inflammatory bowel disease.
Conflict of interest statement
J. S. Alexander is currently receiving a grant (W81XWH-11-1-0577, Lymphatic Vascular-based Therapy in IBD) from the Department of Defense. I. Tsunoda is supported by the National Institute of General Sciences COBRE Grant (P30-GM110703). F. Becker is supported by a fellowship grant from Abbvie Corporation (16,165) and the German Research Foundation (DFG, BE 5619/1-1). The remaining authors have no conflicts of interest to disclose.
Figures


Similar articles
-
Dextran sulfate sodium-induced acute colitis impairs dermal lymphatic function in mice.World J Gastroenterol. 2015 Dec 7;21(45):12767-77. doi: 10.3748/wjg.v21.i45.12767. World J Gastroenterol. 2015. PMID: 26668501 Free PMC article.
-
Platelet interaction with lymphatics aggravates intestinal inflammation by suppressing lymphangiogenesis.Am J Physiol Gastrointest Liver Physiol. 2016 Aug 1;311(2):G276-85. doi: 10.1152/ajpgi.00455.2015. Epub 2016 Jun 16. Am J Physiol Gastrointest Liver Physiol. 2016. PMID: 27313177
-
COMP-angiopoietin-1 ameliorates inflammation-induced lymphangiogenesis in dextran sulfate sodium (DSS)-induced colitis model.J Mol Med (Berl). 2018 May;96(5):459-467. doi: 10.1007/s00109-018-1633-x. Epub 2018 Apr 2. J Mol Med (Berl). 2018. PMID: 29610929 Free PMC article.
-
Emerging roles of lymphatics in inflammatory bowel disease.Ann N Y Acad Sci. 2010 Oct;1207 Suppl 1:E75-85. doi: 10.1111/j.1749-6632.2010.05757.x. Ann N Y Acad Sci. 2010. PMID: 20961310 Review.
-
FOXC2 transcription factor: a novel regulator of lymphangiogenesis.Lymphology. 2011 Mar;44(1):35-41. Lymphology. 2011. PMID: 21667821 Review.
Cited by
-
The Role of the Lymphatic System in the Pathogenesis and Treatment of Inflammatory Bowel Disease.Int J Mol Sci. 2022 Feb 6;23(3):1854. doi: 10.3390/ijms23031854. Int J Mol Sci. 2022. PMID: 35163775 Free PMC article. Review.
-
A zebrafish model of inflammatory lymphangiogenesis.Biol Open. 2015 Sep 14;4(10):1270-80. doi: 10.1242/bio.013540. Biol Open. 2015. PMID: 26369931 Free PMC article.
-
IL-1β reduces tonic contraction of mesenteric lymphatic muscle cells, with the involvement of cycloxygenase-2 and prostaglandin E2.Br J Pharmacol. 2015 Aug;172(16):4038-51. doi: 10.1111/bph.13194. Epub 2015 Jul 6. Br J Pharmacol. 2015. PMID: 25989136 Free PMC article.
-
Cancer and lymphatic marker FOXC2 drives wound healing and fibrotic tissue formation.Front Physiol. 2024 Oct 15;15:1427113. doi: 10.3389/fphys.2024.1427113. eCollection 2024. Front Physiol. 2024. PMID: 39473610 Free PMC article.
-
The Lymphatic Vasculature in the 21st Century: Novel Functional Roles in Homeostasis and Disease.Cell. 2020 Jul 23;182(2):270-296. doi: 10.1016/j.cell.2020.06.039. Cell. 2020. PMID: 32707093 Free PMC article. Review.
References
-
- Burisch J, Jess T, Martinato M, et al. The burden of inflammatory bowel disease in Europe. J Crohns Colitis. 2013;7:322–337. - PubMed
-
- Baumgart DC, Carding SR. Inflammatory bowel disease: cause and immunobiology. Lancet. 2007;369:1627–1640. - PubMed
-
- Baumgart DC, Sandborn WJ. Inflammatory bowel disease: clinical aspects and established and evolving therapies. Lancet. 2007;369:1641–1657. - PubMed
-
- Chidlow JH, Jr, Shukla D, Grisham MB, et al. Pathogenic angiogenesis in IBD and experimental colitis: new ideas and therapeutic avenues. Am J Physiol Gastrointest Liver Physiol. 2007;293:G5–G18. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

