Biomarkers on patient T cells diagnose active tuberculosis and monitor treatment response
- PMID: 25822019
- PMCID: PMC4598074
- DOI: 10.1172/JCI77990
Biomarkers on patient T cells diagnose active tuberculosis and monitor treatment response
Erratum in
-
Biomarkers on patient T cells diagnose active tuberculosis and monitor treatment response.J Clin Invest. 2015 Sep;125(9):3723. doi: 10.1172/JCI83279. Epub 2015 Sep 1. J Clin Invest. 2015. PMID: 26325038 Free PMC article. No abstract available.
Abstract
Background: The identification and treatment of individuals with tuberculosis (TB) is a global public health priority. Accurate diagnosis of pulmonary active TB (ATB) disease remains challenging and relies on extensive medical evaluation and detection of Mycobacterium tuberculosis (Mtb) in the patient's sputum. Further, the response to treatment is monitored by sputum culture conversion, which takes several weeks for results. Here, we sought to identify blood-based host biomarkers associated with ATB and hypothesized that immune activation markers on Mtb-specific CD4+ T cells would be associated with Mtb load in vivo and could thus provide a gauge of Mtb infection.
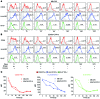
Methods: Using polychromatic flow cytometry, we evaluated the expression of immune activation markers on Mtb-specific CD4+ T cells from individuals with asymptomatic latent Mtb infection (LTBI) and ATB as well as from ATB patients undergoing anti-TB treatment.
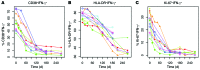
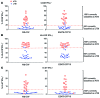
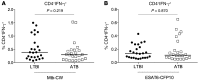
Results: Frequencies of Mtb-specific IFN-γ+CD4+ T cells that expressed immune activation markers CD38 and HLA-DR as well as intracellular proliferation marker Ki-67 were substantially higher in subjects with ATB compared with those with LTBI. These markers accurately classified ATB and LTBI status, with cutoff values of 18%, 60%, and 5% for CD38+IFN-γ+, HLA-DR+IFN-γ+, and Ki-67+IFN-γ+, respectively, with 100% specificity and greater than 96% sensitivity. These markers also distinguished individuals with untreated ATB from those who had successfully completed anti-TB treatment and correlated with decreasing mycobacterial loads during treatment.
Conclusion: We have identified host blood-based biomarkers on Mtb-specific CD4+ T cells that discriminate between ATB and LTBI and provide a set of tools for monitoring treatment response and cure.
Trial registration: Registration is not required for observational studies.
Funding: This study was funded by Emory University, the NIH, and the Yerkes National Primate Center.
Figures




References
-
- Davies PD, Pai M. The diagnosis and misdiagnosis of tuberculosis. Int J Tuberc Lung Dis. 2008;12(11):1226–1234. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

