CCN1 induces hepatic ductular reaction through integrin αvβ₅-mediated activation of NF-κB
- PMID: 25822023
- PMCID: PMC4463205
- DOI: 10.1172/JCI79327
CCN1 induces hepatic ductular reaction through integrin αvβ₅-mediated activation of NF-κB
Abstract
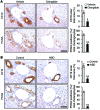
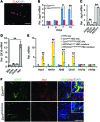
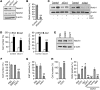
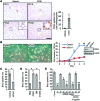
Liver cholestatic diseases, which stem from diverse etiologies, result in liver toxicity and fibrosis and may progress to cirrhosis and liver failure. We show that CCN1 (also known as CYR61), a matricellular protein that dampens and resolves liver fibrosis, also mediates cholangiocyte proliferation and ductular reaction, which are repair responses to cholestatic injury. In cholangiocytes, CCN1 activated NF-κB through integrin αvβ5/αvβ3, leading to Jag1 expression, JAG1/NOTCH signaling, and cholangiocyte proliferation. CCN1 also induced Jag1 expression in hepatic stellate cells, whereupon they interacted with hepatic progenitor cells to promote their differentiation into cholangiocytes. Administration of CCN1 protein or soluble JAG1 induced cholangiocyte proliferation in mice, which was blocked by inhibitors of NF-κB or NOTCH signaling. Knock-in mice expressing a CCN1 mutant that is unable to bind αvβ5/αvβ3 were impaired in ductular reaction, leading to massive hepatic necrosis and mortality after bile duct ligation (BDL), whereas treatment of these mice with soluble JAG1 rescued ductular reaction and reduced hepatic necrosis and mortality. Blockade of integrin αvβ5/αvβ3, NF-κB, or NOTCH signaling in WT mice also resulted in defective ductular reaction after BDL. These findings demonstrate that CCN1 induces cholangiocyte proliferation and ductular reaction and identify CCN1/αvβ5/NF-κB/JAG1 as a critical axis for biliary injury repair.
Figures





Similar articles
-
Matricellular protein CCN1 promotes regression of liver fibrosis through induction of cellular senescence in hepatic myofibroblasts.Mol Cell Biol. 2013 May;33(10):2078-90. doi: 10.1128/MCB.00049-13. Epub 2013 Mar 18. Mol Cell Biol. 2013. PMID: 23508104 Free PMC article.
-
Sortilin Deficiency Reduces Ductular Reaction, Hepatocyte Apoptosis, and Liver Fibrosis in Cholestatic-Induced Liver Injury.Am J Pathol. 2017 Jan;187(1):122-133. doi: 10.1016/j.ajpath.2016.09.005. Epub 2016 Nov 11. Am J Pathol. 2017. PMID: 27842214
-
Prominin-1-expressing hepatic progenitor cells induce fibrogenesis in murine cholestatic liver injury.Physiol Rep. 2020 Jul;8(14):e14508. doi: 10.14814/phy2.14508. Physiol Rep. 2020. PMID: 32686913 Free PMC article.
-
Significance of CCNs in liver regeneration.J Cell Commun Signal. 2023 Jun;17(2):321-332. doi: 10.1007/s12079-023-00762-x. Epub 2023 May 18. J Cell Commun Signal. 2023. PMID: 37202628 Free PMC article. Review.
-
Unraveling the complexities of fibrosis and ductular reaction in liver disease: pathogenesis, mechanisms, and therapeutic insights.Am J Physiol Cell Physiol. 2024 Mar 1;326(3):C698-C706. doi: 10.1152/ajpcell.00486.2023. Epub 2023 Dec 18. Am J Physiol Cell Physiol. 2024. PMID: 38105754 Free PMC article. Review.
Cited by
-
Smad4 restricts injury-provoked biliary proliferation and carcinogenesis.Dis Model Mech. 2024 Jun 1;17(6):dmm050358. doi: 10.1242/dmm.050358. Epub 2024 Feb 28. Dis Model Mech. 2024. PMID: 38415925 Free PMC article.
-
Cellular communication network factor 1-stimulated liver macrophage efferocytosis drives hepatic stellate cell activation and liver fibrosis.Hepatol Commun. 2022 Oct;6(10):2798-2811. doi: 10.1002/hep4.2057. Epub 2022 Aug 5. Hepatol Commun. 2022. PMID: 35929736 Free PMC article.
-
Bioinformatic analysis identified novel candidate genes with the potentials for diagnostic blood testing of primary biliary cholangitis.PLoS One. 2023 Oct 16;18(10):e0292998. doi: 10.1371/journal.pone.0292998. eCollection 2023. PLoS One. 2023. PMID: 37844121 Free PMC article.
-
The Prognostic Role of SOCS3 and A20 in Human Cholangiocarcinoma.PLoS One. 2015 Oct 20;10(10):e0141165. doi: 10.1371/journal.pone.0141165. eCollection 2015. PLoS One. 2015. PMID: 26485275 Free PMC article.
-
Identification of acquired Notch3 dependency in metastatic Head and Neck Cancer.Commun Biol. 2023 May 18;6(1):538. doi: 10.1038/s42003-023-04828-9. Commun Biol. 2023. PMID: 37202533 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

