Noninvasive detection of tumor-infiltrating T cells by PET reporter imaging
- PMID: 25822024
- PMCID: PMC4463193
- DOI: 10.1172/JCI77326
Noninvasive detection of tumor-infiltrating T cells by PET reporter imaging
Abstract
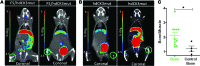
Adoptive transfer of tumor-reactive T cells can successfully reduce tumor burden; however, in rare cases, lethal on-target/off-tumor effects have been reported. A noninvasive method to track engineered cells with high sensitivity and resolution would allow observation of correct cell homing and/or identification of dangerous off-target locations in preclinical and clinical applications. Human deoxycytidine kinase triple mutant (hdCK3mut) is a nonimmunogenic PET reporter that was previously shown to be an effective tool to monitor whole-body hematopoiesis. Here, we engineered a construct in which hdCK3mut is coexpressed with the anti-melanoma T cell receptor F5, introduced this construct into human CD34 cells or PBMCs, and evaluated this approach in multiple immunotherapy models. Expression of hdCK3mut allowed engrafted cells to be visualized within recipient bone marrow, while accumulation of [18F]-L-FMAU in hdCK3mut-expressing T cells permitted detection of intratumoral homing. Animals that received T cells coexpressing hdCK3mut and the anti-melanoma T cell receptor had demonstrably higher signals in HLA-matched tumors compared with those in animals that received cells solely expressing hdCK3mut. Engineered T cells caused cytotoxicity in HLA/antigen-matched tumors and induced IFN-γ production and activation. Moreover, hdCK3mut permitted simultaneous monitoring of engraftment and tumor infiltration, without affecting T cell function. Our findings suggest that hdCK3mut reporter imaging can be applied in clinical immunotherapies for whole-body detection of engineered cell locations.
Figures







Similar articles
-
Long-term in vivo monitoring of mouse and human hematopoietic stem cell engraftment with a human positron emission tomography reporter gene.Proc Natl Acad Sci U S A. 2013 Jan 29;110(5):1857-62. doi: 10.1073/pnas.1221840110. Epub 2013 Jan 14. Proc Natl Acad Sci U S A. 2013. PMID: 23319634 Free PMC article.
-
T-cell receptor gene therapy in human melanoma-bearing immune-deficient mice: human but not mouse T cells recapitulate outcome of clinical studies.Hum Gene Ther. 2012 Feb;23(2):187-201. doi: 10.1089/hum.2010.126. Epub 2011 Dec 2. Hum Gene Ther. 2012. PMID: 21958294
-
Potential use of T cell receptor genes to modify hematopoietic stem cells for the gene therapy of cancer.Pathol Oncol Res. 1999;5(1):3-15. doi: 10.1053/paor.1999.0003. Pathol Oncol Res. 1999. PMID: 10079371 Review.
-
Simultaneous generation of CD8+ and CD4+ melanoma-reactive T cells by retroviral-mediated transfer of a single T-cell receptor.Cancer Res. 2005 Feb 15;65(4):1570-6. doi: 10.1158/0008-5472.CAN-04-2076. Cancer Res. 2005. PMID: 15735047
-
Chimeric antigen receptor engineering: a right step in the evolution of adoptive cellular immunotherapy.Int Rev Immunol. 2015 Mar;34(2):154-87. doi: 10.3109/08830185.2015.1018419. Int Rev Immunol. 2015. PMID: 25901860 Review.
Cited by
-
In vivo Imaging Technologies to Monitor the Immune System.Front Immunol. 2020 Jun 2;11:1067. doi: 10.3389/fimmu.2020.01067. eCollection 2020. Front Immunol. 2020. PMID: 32582173 Free PMC article. Review.
-
In vivo CART cell imaging: Paving the way for success in CART cell therapy.Mol Ther Oncolytics. 2021 Mar 5;20:625-633. doi: 10.1016/j.omto.2021.03.003. eCollection 2021 Mar 26. Mol Ther Oncolytics. 2021. PMID: 33816781 Free PMC article. Review.
-
An Effective Immuno-PET Imaging Method to Monitor CD8-Dependent Responses to Immunotherapy.Cancer Res. 2016 Jan 1;76(1):73-82. doi: 10.1158/0008-5472.CAN-15-1707. Epub 2015 Nov 16. Cancer Res. 2016. PMID: 26573799 Free PMC article.
-
Improvements and Limitations of Humanized Mouse Models for HIV Research: NIH/NIAID "Meet the Experts" 2015 Workshop Summary.AIDS Res Hum Retroviruses. 2016 Feb;32(2):109-19. doi: 10.1089/AID.2015.0258. AIDS Res Hum Retroviruses. 2016. PMID: 26670361 Free PMC article.
-
Applications of nuclear-based imaging in gene and cell therapy: probe considerations.Mol Ther Oncolytics. 2021 Feb 4;20:447-458. doi: 10.1016/j.omto.2021.01.017. eCollection 2021 Mar 26. Mol Ther Oncolytics. 2021. PMID: 33718593 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

