C. elegans outside the Petri dish
- PMID: 25822066
- PMCID: PMC4373675
- DOI: 10.7554/eLife.05849
C. elegans outside the Petri dish
Abstract
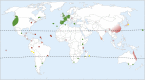
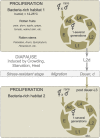
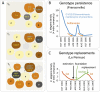
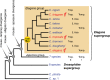
The roundworm Caenorhabditis elegans has risen to the status of a top model organism for biological research in the last fifty years. Among laboratory animals, this tiny nematode is one of the simplest and easiest organisms to handle. And its life outside the laboratory is beginning to be unveiled. Like other model organisms, C. elegans has a boom-and-bust lifestyle. It feasts on ephemeral bacterial blooms in decomposing fruits and stems. After resource depletion, its young larvae enter a migratory diapause stage, called the dauer. Organisms known to be associated with C. elegans include migration vectors (such as snails, slugs and isopods) and pathogens (such as microsporidia, fungi, bacteria and viruses). By deepening our understanding of the natural history of C. elegans, we establish a broader context and improved tools for studying its biology.
Keywords: C. elegans; ecology; evolution; evolutionary biology; genomics; natural history; the natural history of model organisms.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures

Similar articles
-
Population dynamics and habitat sharing of natural populations of Caenorhabditis elegans and C. briggsae.BMC Biol. 2012 Jun 25;10:59. doi: 10.1186/1741-7007-10-59. BMC Biol. 2012. PMID: 22731941 Free PMC article.
-
The worm in the world and the world in the worm.BMC Biol. 2012 Jun 25;10:57. doi: 10.1186/1741-7007-10-57. BMC Biol. 2012. PMID: 22731915 Free PMC article.
-
Genetic analysis of dauer formation in Caenorhabditis briggsae.Genetics. 2007 Oct;177(2):809-18. doi: 10.1534/genetics.107.078857. Epub 2007 Jul 29. Genetics. 2007. PMID: 17660533 Free PMC article.
-
Revising the standard wisdom of C. elegans natural history: ecology of longevity.Sci Aging Knowledge Environ. 2005 Oct 5;2005(40):pe30. doi: 10.1126/sageke.2005.40.pe30. Sci Aging Knowledge Environ. 2005. PMID: 16207928 Free PMC article. Review.
-
The Natural Biotic Environment of Caenorhabditis elegans.Genetics. 2017 May;206(1):55-86. doi: 10.1534/genetics.116.195511. Genetics. 2017. PMID: 28476862 Free PMC article. Review.
Cited by
-
Dehydrated Caenorhabditis elegans Stocks Are Resistant to Multiple Freeze-Thaw Cycles.G3 (Bethesda). 2020 Dec 3;10(12):4505-4512. doi: 10.1534/g3.120.401825. G3 (Bethesda). 2020. PMID: 33033066 Free PMC article.
-
Wrapping culture plates with Parafilm M® increases Caenorhabditis elegans growth.BMC Res Notes. 2019 Dec 19;12(1):818. doi: 10.1186/s13104-019-4854-3. BMC Res Notes. 2019. PMID: 31856898 Free PMC article.
-
Deep sampling of Hawaiian Caenorhabditis elegans reveals high genetic diversity and admixture with global populations.Elife. 2019 Dec 3;8:e50465. doi: 10.7554/eLife.50465. Elife. 2019. PMID: 31793880 Free PMC article.
-
Accumulation of Glycogen and Upregulation of LEA-1 in C. elegans daf-2(e1370) Support Stress Resistance, Not Longevity.Cells. 2022 Jan 12;11(2):245. doi: 10.3390/cells11020245. Cells. 2022. PMID: 35053361 Free PMC article.
-
The impact of species-wide gene expression variation on Caenorhabditis elegans complex traits.Nat Commun. 2022 Jun 16;13(1):3462. doi: 10.1038/s41467-022-31208-4. Nat Commun. 2022. PMID: 35710766 Free PMC article.
References
-
- Abdul Kader N, Côté MG. Isolement, identification et caractérisation de souches québécoises du nématode Caenorhabditis elegans. Fundamental and Applied Nematology. 1996;19:381–389.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

