Remodeling of the residual gastric mucosa after roux-en-y gastric bypass or vertical sleeve gastrectomy in diet-induced obese rats
- PMID: 25822172
- PMCID: PMC4379088
- DOI: 10.1371/journal.pone.0121414
Remodeling of the residual gastric mucosa after roux-en-y gastric bypass or vertical sleeve gastrectomy in diet-induced obese rats
Abstract
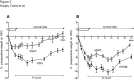
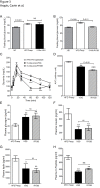
Whereas the remodeling of intestinal mucosa after bariatric surgeries has been the matter of numerous studies to our knowledge, very few reported on the remodeling of the residual gastric mucosa. In this study, we analyzed remodeling of gastric mucosa after Roux-en-Y gastric bypass (RYGB) and vertical sleeve gastrectomy (VSG) in rats. Diet-induced obese rats were subjected to RYGB, VSG or sham surgical procedures. All animals were assessed for food intake, body-weight, fasting blood, metabolites and hormones profiling, as well as insulin and glucose tolerance tests before and up to 5 weeks post-surgery. Remodeling of gastric tissues was analyzed by routine histology and immunohistochemistry studies, and qRT-PCR analyses of ghrelin and gastrin mRNA levels. In obese rats with impaired glucose tolerance, VSG and RYGB caused substantial weight loss and rats greatly improved their oral glucose tolerance. The remaining gastric mucosa after VSG and gastric pouch (GP) after RYGB revealed a hyperplasia of the mucous neck cells that displayed a strong immunoreactivity for parietal cell H+/K+-ATPase. Ghrelin mRNA levels were reduced by 2-fold in remaining fundic mucosa after VSG and 10-fold in GP after RYGB. In the antrum, gastrin mRNA levels were reduced after VSG in line with the reduced number of gastrin positive cells. This study reports novel and important observations dealing with the remaining gastric mucosa after RYGB and VSG. The data demonstrate, for the first time, a hyperplasia of the mucous neck cells, a transit cell population of the stomach bearing differentiating capacities into zymogenic and peptic cells.
Conflict of interest statement
Figures




References
-
- Sjöström L, Lindroos A-K, Peltonen M, Torgerson J, Bouchard C, Carlsson B, et al. Lifestyle, Diabetes, and Cardiovascular Risk Factors 10 Years after Bariatric Surgery. N Engl J Med. 2004; 351: 2683–2693. - PubMed
-
- Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: A systematic review and meta-analysis. JAMA. 2004; 292: 1724–1737. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

