Novel mechanism of arenavirus-induced liver pathology
- PMID: 25822203
- PMCID: PMC4378851
- DOI: 10.1371/journal.pone.0122839
Novel mechanism of arenavirus-induced liver pathology
Abstract
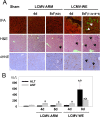
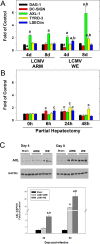
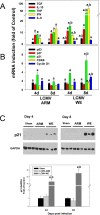
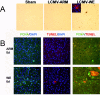
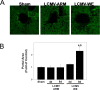
Viral hemorrhagic fevers (VHFs) encompass a group of diseases with cardinal symptoms of fever, hemorrhage, and shock. The liver is a critical mediator of VHF disease pathogenesis and high levels of ALT/AST transaminases in plasma correlate with poor prognosis. In fact, Lassa Fever (LF), the most prevalent VHF in Africa, was initially clinically described as hepatitis. Previous studies in non-human primate (NHP) models also correlated LF pathogenesis with a robust proliferative response in the liver. The purpose of the current study was to gain insight into the mechanism of liver injury and to determine the potential role of proliferation in LF pathogenesis. C57Bl/6J mice were infected with either the pathogenic (for NHPs) strain of lymphocytic choriomeningitis virus (LCMV, the prototypic arenavirus), LCMV-WE, or with the non-pathogenic strain, LCMV-ARM. As expected, LCMV-WE, but not ARM, caused a hepatitis-like infection. LCMV-WE also induced a robust increase in the number of actively cycling hepatocytes. Despite this increase in proliferation, there was no significant difference in liver size between LCMV-WE and LCMV-ARM, suggesting that cell cycle was incomplete. Indeed, cells appeared arrested in the G1 phase and LCMV-WE infection increased the number of hepatocytes that were simultaneously stained for proliferation and apoptosis. LCMV-WE infection also induced expression of a non-conventional virus receptor, AXL-1, from the TAM (TYRO3/AXL/MERTK) family of receptor tyrosine kinases and this expression correlated with proliferation. Taken together, these results shed new light on the mechanism of liver involvement in VHF pathogenesis. Specifically, it is hypothesized that the induction of hepatocyte proliferation contributes to expansion of the infection to parenchymal cells. Elevated levels of plasma transaminases are likely explained, at least in part, by abortive cell cycle arrest induced by the infection. These results may lead to the development of new therapies to prevent VHF progression.
Conflict of interest statement
Figures

References
-
- Crespo G, Marino Z, Navasa M, Forns X (2012) Viral Hepatitis in liver transplantation. Gastroenterology 142: 1373–1383. doi: 10.1053/j.gastro.2012.02.011 - DOI - PubMed
-
- Zhou X, Ramachandran S, Mann M, Popkin DL (2012) Role of Lymphocytic Choriomeningitis Virus (LCMV) in Understanding Viral Immunology: Past, Present and Future. Viruses 4: 2650–2669. doi: 10.3390/v4112650 - DOI - PMC - PubMed
-
- Zinkernagel R (2002) Lymphocytic choriomeningitis virus and immunology. Curr Top Microbiol Immunol 262: 1–5. - PubMed
-
- Buchmeier MJ, Welsh RM, Dutko FJ, Oldstone MBA (1980) The virology and immunobiology of lymphocytic choriomeningitis virus infection. Adv Immunol 30: 275–331. - PubMed
-
- Salvato MS, Clegg JCS, Buchmeier MJ, Charrel RN, Gonzalez JP, et al. (2012) Family Arenaviridae In: King A.M.Q., Adams M.J., Carstens E.B., Lefkowitz E.J. (Eds.), Virus Taxonomy, IXth Report of the ICTV. Elsevier/Academic Press, London: 715–723.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

