Thyroxine differentially modulates the peripheral clock: lessons from the human hair follicle
- PMID: 25822259
- PMCID: PMC4379003
- DOI: 10.1371/journal.pone.0121878
Thyroxine differentially modulates the peripheral clock: lessons from the human hair follicle
Abstract
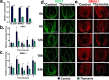
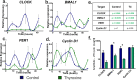
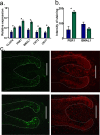
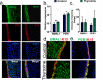
The human hair follicle (HF) exhibits peripheral clock activity, with knock-down of clock genes (BMAL1 and PER1) prolonging active hair growth (anagen) and increasing pigmentation. Similarly, thyroid hormones prolong anagen and stimulate pigmentation in cultured human HFs. In addition they are recognized as key regulators of the central clock that controls circadian rhythmicity. Therefore, we asked whether thyroxine (T4) also influences peripheral clock activity in the human HF. Over 24 hours we found a significant reduction in protein levels of BMAL1 and PER1, with their transcript levels also decreasing significantly. Furthermore, while all clock genes maintained their rhythmicity in both the control and T4 treated HFs, there was a significant reduction in the amplitude of BMAL1 and PER1 in T4 (100 nM) treated HFs. Accompanying this, cell-cycle progression marker Cyclin D1 was also assessed appearing to show an induced circadian rhythmicity by T4 however, this was not significant. Contrary to short term cultures, after 6 days, transcript and/or protein levels of all core clock genes (BMAL1, PER1, clock, CRY1, CRY2) were up-regulated in T4 treated HFs. BMAL1 and PER1 mRNA was also up-regulated in the HF bulge, the location of HF epithelial stem cells. Together this provides the first direct evidence that T4 modulates the expression of the peripheral molecular clock. Thus, patients with thyroid dysfunction may also show a disordered peripheral clock, which raises the possibility that short term, pulsatile treatment with T4 might permit one to modulate circadian activity in peripheral tissues as a target to treat clock-related disease.
Conflict of interest statement
Figures
Similar articles
-
A meeting of two chronobiological systems: circadian proteins Period1 and BMAL1 modulate the human hair cycle clock.J Invest Dermatol. 2014 Mar;134(3):610-619. doi: 10.1038/jid.2013.366. Epub 2013 Sep 4. J Invest Dermatol. 2014. PMID: 24005054
-
The peripheral clock regulates human pigmentation.J Invest Dermatol. 2015 Apr;135(4):1053-1064. doi: 10.1038/jid.2014.442. Epub 2014 Oct 13. J Invest Dermatol. 2015. PMID: 25310406
-
Hypophysectomy abolishes rhythms in rat thyroid hormones but not in the thyroid clock.J Endocrinol. 2017 Jun;233(3):209-216. doi: 10.1530/JOE-17-0111. Epub 2017 Mar 27. J Endocrinol. 2017. PMID: 28348112 Free PMC article.
-
Circadian modification network of a core clock driver BMAL1 to harmonize physiology from brain to peripheral tissues.Neurochem Int. 2018 Oct;119:11-16. doi: 10.1016/j.neuint.2017.12.013. Epub 2018 Jan 3. Neurochem Int. 2018. PMID: 29305918 Review.
-
[Molecular mechanisms of circadian clock functioning].Ukr Biokhim Zh (1999). 2011 May-Jun;83(3):5-24. Ukr Biokhim Zh (1999). 2011. PMID: 21888051 Review. Ukrainian.
Cited by
-
Exploring phylogeny to find the function of sleep.Nat Rev Neurosci. 2019 Feb;20(2):109-116. doi: 10.1038/s41583-018-0098-9. Nat Rev Neurosci. 2019. PMID: 30573905 Review.
-
The Wnt/β-catenin signaling pathway is involved in regulating feather growth of embryonic chicks.Poult Sci. 2020 May;99(5):2315-2323. doi: 10.1016/j.psj.2020.01.002. Epub 2020 Mar 18. Poult Sci. 2020. PMID: 32359566 Free PMC article.
-
RNAseq shows an all-pervasive day-night rhythm in the transcriptome of the pacemaker of the heart.Sci Rep. 2021 Feb 11;11(1):3565. doi: 10.1038/s41598-021-82202-7. Sci Rep. 2021. PMID: 33574422 Free PMC article.
-
Photoperiod Management in Farm Animal Husbandry: A Review.Animals (Basel). 2025 Feb 18;15(4):591. doi: 10.3390/ani15040591. Animals (Basel). 2025. PMID: 40003072 Free PMC article. Review.
-
Topical L-thyroxine: The Cinderella among hormones waiting to dance on the floor of dermatological therapy?Exp Dermatol. 2020 Sep;29(9):910-923. doi: 10.1111/exd.14156. Epub 2020 Aug 28. Exp Dermatol. 2020. PMID: 32682336 Free PMC article. Review.
References
-
- Geyfman M, Kumar V, Liu Q, Ruiz R, Gordon W, Espitia F, et al. (2012) Brain and muscle Arnt-like protein-1 (BMAL1) controls circadian cell proliferation and susceptibility to UVB-induced DNA damage in the epidermis. Proc Natl Acad Sci U S A 109: 11758–11763. 10.1073/pnas.1209592109 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

