Conantokin-G attenuates detrimental effects of NMDAR hyperactivity in an ischemic rat model of stroke
- PMID: 25822337
- PMCID: PMC4379059
- DOI: 10.1371/journal.pone.0122840
Conantokin-G attenuates detrimental effects of NMDAR hyperactivity in an ischemic rat model of stroke
Abstract
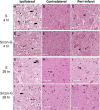
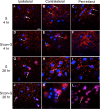
The neuroprotective activity of conantokin-G (con-G), a naturally occurring antagonist of N-methyl-D-aspartate receptors (NMDAR), was neurologically and histologically compared in the core and peri-infarct regions after ischemia/reperfusion brain injury in male Sprague-Dawley rats. The contralateral regions served as robust internal controls. Intrathecal injection of con-G, post-middle carotid artery occlusion (MCAO), caused a dramatic decrease in brain infarct size and swelling at 4 hr, compared to 26 hr, and significant recovery of neurological deficits was observed at 26 hr. Administration of con-G facilitated neuronal recovery in the peri-infarct regions as observed by decreased neurodegeneration and diminished calcium microdeposits at 4 hr and 26 hr. Intact Microtubule Associated Protein (MAP2) staining and neuronal cytoarchitecture was observed in the peri-infarct regions of con-G treated rats at both timepoints. Con-G restored localization of GluN1 and GluN2B subunits in the neuronal soma, but not that of GluN2A, which was perinuclear in the peri-infarct regions at 4 hr and 26 hr. This suggests that molecular targeting of the GluN2B subunit has potential for reducing detrimental consequences of ischemia. Overall, the data demonstrated that stroke-induced NMDAR excitoxicity is ameliorated by con-G-mediated repair of neurological and neuroarchitectural deficits, as well as by reconstituting neuronal localization of GluN1 and GluN2B subunits in the peri-infarct region of the stroked brain.
Conflict of interest statement
Figures







References
-
- Arundine M, Tymianski M (2003) Molecular mechanisms of calcium-dependent neurodegeneration in excitotoxicity. Cell Calcium 34:325–327. - PubMed
-
- Hollmann M, Heinemann S (1994) Cloned glutamate receptors. Annu Rev Neurosci 17:31–108. - PubMed
-
- Loftis JM, Janowsky A (2003) The N-methyl-D-aspartate receptor subunit NR2B: localization, functional properties, regulation, and clinical implications. Pharmacol Ther 97: 55–85. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

