Epidemiology of adverse drug reactions in Europe: a review of recent observational studies
- PMID: 25822400
- PMCID: PMC4412588
- DOI: 10.1007/s40264-015-0281-0
Epidemiology of adverse drug reactions in Europe: a review of recent observational studies
Abstract
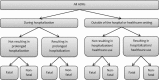
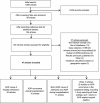
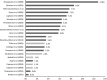
Adverse drug reactions (ADRs) cause considerable mortality and morbidity but no recent reviews are currently available for the European region. Therefore, we performed a review of all epidemiological studies quantifying ADRs in a European setting that were published between 1 January 2000 and 3 September 2014. Included studies assessed the number of patients who were admitted to hospital due to an ADR, studies that assessed the number of patients who developed an ADR during hospitalization, and studies that measured ADRs in the outpatient setting. In total, 47 articles were included in the final review. The median percentage of hospital admissions due to an ADR was 3.5 %, based on 22 studies, and the median percentage of patients who experienced an ADR during hospitalization was 10.1 %, based on 13 studies. Only five studies were found that assessed ADRs occurring in the outpatient setting. These results indicate that the occurrence of ADRs in the European hospital setting-both ADRs that result in hospitalization and ADRs that occur during the hospital stay-is significant. Furthermore, the limited number of studies that were performed in the outpatient setting identify a lack of information regarding the epidemiology of ADRs in this setting.
Figures

References
-
- European Commission. Proposal for a regulation amending, as regards pharmacovigilance of medicinal products for human use. Regulation (EC) No 726/2004. Impact assessment. 2008. Available at: http://ec.europa.eu/health/files/pharmacos/pharmpack_12_2008/pharmacovig.... Accessed 3 Sept 2014.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

