Human gastroenteropancreatic expression of melatonin and its receptors MT1 and MT2
- PMID: 25822611
- PMCID: PMC4378860
- DOI: 10.1371/journal.pone.0120195
Human gastroenteropancreatic expression of melatonin and its receptors MT1 and MT2
Abstract
Background and aim: The largest source of melatonin, according to animal studies, is the gastrointestinal (GI) tract but this is not yet thoroughly characterized in humans. This study aims to map the expression of melatonin and its two receptors in human GI tract and pancreas using microarray analysis and immunohistochemistry.
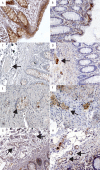
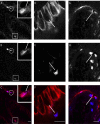
Method: Gene expression data from normal intestine and pancreas and inflamed colon tissue due to ulcerative colitis were analyzed for expression of enzymes relevant for serotonin and melatonin production and their receptors. Sections from paraffin-embedded normal tissue from 42 individuals, representing the different parts of the GI tract (n=39) and pancreas (n=3) were studied with immunohistochemistry using antibodies with specificity for melatonin, MT1 and MT2 receptors and serotonin.
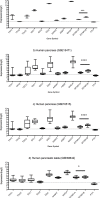
Results: Enzymes needed for production of melatonin are expressed in both GI tract and pancreas tissue. Strong melatonin immunoreactivity (IR) was seen in enterochromaffin (EC) cells partially co-localized with serotonin IR. Melatonin IR was also seen in pancreas islets. MT1 and MT2 IR were both found in the intestinal epithelium, in the submucosal and myenteric plexus, and in vessels in the GI tract as well as in pancreatic islets. MT1 and MT2 IR was strongest in the epithelium of the large intestine. In the other cell types, both MT2 gene expression and IR were generally elevated compared to MT1. Strong MT2, IR was noted in EC cells but not MT1 IR. Changes in gene expression that may result in reduced levels of melatonin were seen in relation to inflammation.
Conclusion: Widespread gastroenteropancreatic expression of melatonin and its receptors in the GI tract and pancreas is in agreement with the multiple roles ascribed to melatonin, which include regulation of gastrointestinal motility, epithelial permeability as well as enteropancreatic cross-talk with plausible impact on metabolic control.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

