Spatiotemporal analysis of hepatitis C virus infection
- PMID: 25822891
- PMCID: PMC4378894
- DOI: 10.1371/journal.ppat.1004758
Spatiotemporal analysis of hepatitis C virus infection
Abstract
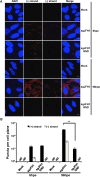
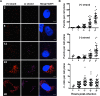
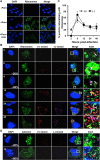
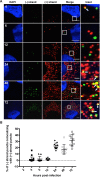
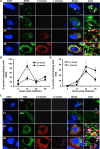
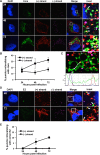
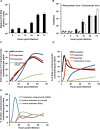
Hepatitis C virus (HCV) entry, translation, replication, and assembly occur with defined kinetics in distinct subcellular compartments. It is unclear how HCV spatially and temporally regulates these events within the host cell to coordinate its infection. We have developed a single molecule RNA detection assay that facilitates the simultaneous visualization of HCV (+) and (-) RNA strands at the single cell level using high-resolution confocal microscopy. We detect (+) strand RNAs as early as 2 hours post-infection and (-) strand RNAs as early as 4 hours post-infection. Single cell levels of (+) and (-) RNA vary considerably with an average (+):(-) RNA ratio of 10 and a range from 1-35. We next developed microscopic assays to identify HCV (+) and (-) RNAs associated with actively translating ribosomes, replication, virion assembly and intracellular virions. (+) RNAs display a defined temporal kinetics, with the majority of (+) RNAs associated with actively translating ribosomes at early times of infection, followed by a shift to replication and then virion assembly. (-) RNAs have a strong colocalization with NS5A, but not NS3, at early time points that correlate with replication compartment formation. At later times, only ~30% of the replication complexes appear to be active at a given time, as defined by (-) strand colocalization with either (+) RNA, NS3, or NS5A. While both (+) and (-) RNAs colocalize with the viral proteins NS3 and NS5A, only the plus strand preferentially colocalizes with the viral envelope E2 protein. These results suggest a defined spatiotemporal regulation of HCV infection with highly varied replication efficiencies at the single cell level. This approach can be applicable to all plus strand RNA viruses and enables unprecedented sensitivity for studying early events in the viral life cycle.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Lohmann V, Korner F, Koch J, Herian U, Theilmann L, et al. (1999) Replication of subgenomic hepatitis C virus RNAs in a hepatoma cell line. Science 285: 110–113. - PubMed
-
- Blight KJ, Kolykhalov AA, Rice CM (2000) Efficient initiation of HCV RNA replication in cell culture. Science 290: 1972–1974. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

