Deficiency of angulin-2/ILDR1, a tricellular tight junction-associated membrane protein, causes deafness with cochlear hair cell degeneration in mice
- PMID: 25822906
- PMCID: PMC4378975
- DOI: 10.1371/journal.pone.0120674
Deficiency of angulin-2/ILDR1, a tricellular tight junction-associated membrane protein, causes deafness with cochlear hair cell degeneration in mice
Abstract
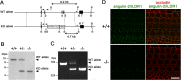
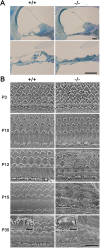
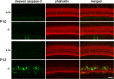
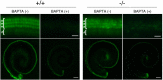
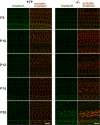
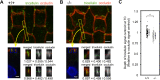
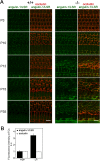
Tricellular tight junctions seal the extracellular spaces of tricellular contacts, where the vertices of three epithelial cells meet, and are required for the establishment of a strong barrier function of the epithelial cellular sheet. Angulins and tricellulin are known as specific protein components of tricellular tight junctions, where angulins recruit tricellulin. Mutations in the genes encoding angulin-2/ILDR1 and tricellulin have been reported to cause human hereditary deafness DFNB42 and DFNB49, respectively. To investigate the pathogenesis of DFNB42, we analyzed mice with a targeted disruption of Ildr1, which encodes angulin-2/ILDR1. Ildr1 null mice exhibited profound deafness. Hair cells in the cochlea of Ildr1 null mice develop normally, but begin to degenerate by two weeks after birth. Tricellulin localization at tricellular contacts of the organ of Corti in the cochlea was retained in Ildr1 null mice, but its distribution along the depth of tricellular contacts was affected. Interestingly, compensatory tricellular contact localization of angulin-1/LSR was observed in the organ of Corti in Ildr1 null mice although it was hardly detected in the organ of Corti in wild-type mice. The onset of hair cell degeneration in Ildr1 null mice was earlier than that in the reported Tric mutant mice, which mimic one of the tricellulin mutations in DFNB49 deafness. These results indicate that the angulin-2/ILDR1 deficiency causes the postnatal degenerative loss of hair cells in the cochlea, leading to human deafness DFNB42. Our data also suggest that angulin family proteins have distinct functions in addition to their common roles of tricellulin recruitment and that the function of angulin-2/ILDR1 for hearing cannot be substituted by angulin-1/LSR.
Conflict of interest statement
Figures

Similar articles
-
Analysis of the 'angulin' proteins LSR, ILDR1 and ILDR2--tricellulin recruitment, epithelial barrier function and implication in deafness pathogenesis.J Cell Sci. 2013 Feb 15;126(Pt 4):966-77. doi: 10.1242/jcs.116442. Epub 2012 Dec 13. J Cell Sci. 2013. PMID: 23239027
-
ILDR1 null mice, a model of human deafness DFNB42, show structural aberrations of tricellular tight junctions and degeneration of auditory hair cells.Hum Mol Genet. 2015 Feb 1;24(3):609-24. doi: 10.1093/hmg/ddu474. Epub 2014 Sep 12. Hum Mol Genet. 2015. PMID: 25217574 Free PMC article.
-
[Molecular organization of tricellular tight junctions].Yakugaku Zasshi. 2014;134(5):615-21. doi: 10.1248/yakushi.14-00006-1. Yakugaku Zasshi. 2014. PMID: 24790043 Review. Japanese.
-
Downsloping high-frequency hearing loss due to inner ear tricellular tight junction disruption by a novel ILDR1 mutation in the Ig-like domain.PLoS One. 2015 Feb 10;10(2):e0116931. doi: 10.1371/journal.pone.0116931. eCollection 2015. PLoS One. 2015. PMID: 25668204 Free PMC article.
-
Tricellular Tight Junctions in the Inner Ear.Biomed Res Int. 2016;2016:6137541. doi: 10.1155/2016/6137541. Epub 2016 Apr 18. Biomed Res Int. 2016. PMID: 27195292 Free PMC article. Review.
Cited by
-
Striatin Is Required for Hearing and Affects Inner Hair Cells and Ribbon Synapses.Front Cell Dev Biol. 2020 Jul 15;8:615. doi: 10.3389/fcell.2020.00615. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32766247 Free PMC article.
-
Population dynamics of potentially harmful haplotypes: a pedigree analysis.BMC Genomics. 2024 May 16;25(1):487. doi: 10.1186/s12864-024-10407-x. BMC Genomics. 2024. PMID: 38755557 Free PMC article.
-
Discovering the Unexpected with the Utilization of NGS in Diagnostics of Non-syndromic Hearing Loss Disorders: The Family Case of ILDR1-Dependent Hearing Loss Disorder.Front Genet. 2017 Jun 30;8:95. doi: 10.3389/fgene.2017.00095. eCollection 2017. Front Genet. 2017. PMID: 28713423 Free PMC article.
-
ILDR1 promotes influenza A virus replication through binding to PLSCR1.Sci Rep. 2022 May 20;12(1):8515. doi: 10.1038/s41598-022-12598-3. Sci Rep. 2022. PMID: 35595813 Free PMC article.
-
Cell Biology of Tight Junction Barrier Regulation and Mucosal Disease.Cold Spring Harb Perspect Biol. 2018 Jan 2;10(1):a029314. doi: 10.1101/cshperspect.a029314. Cold Spring Harb Perspect Biol. 2018. PMID: 28507021 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

