Overexpression of AtTTP affects ARF17 expression and leads to male sterility in Arabidopsis
- PMID: 25822980
- PMCID: PMC4378849
- DOI: 10.1371/journal.pone.0117317
Overexpression of AtTTP affects ARF17 expression and leads to male sterility in Arabidopsis
Abstract
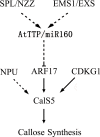
Callose synthesis is critical for the formation of the pollen wall pattern. CalS5 is thought to be the major synthethase for the callose wall. In the Arabidopsis anther, ARF17 regulates the expression of CalS5 and is the target of miR160. Plants expressing miR160-resistant ARF17 (35S:5mARF17 lines) with increased ARF17 mRNA levels display male sterility. Here we report a zinc finger family gene, AtTTP, which is involved in miR160 maturation and callose synthesis in Arabidopsis. AtTTP is expressed in microsporocytes, tetrads and tapetal cells in the anther. Over-expression lines of AtTTP (AtTTP-OE line) exhibited reduced male fertility. CalS5 expression was tremendously reduced and the tetrad callose wall became much thinner in the AtTTP-OE line. Northern blotting hybridization and quantitative RT-PCR analysis revealed that miR160 was decreased, while the expression of ARF17 was increased in the AtTTP-OE line. Based on these results, we propose that AtTTP associates with miR160 in order to regulate the ARF17 expression needed for callose synthesis and pollen wall formation.
Conflict of interest statement
Figures





Similar articles
-
Fine regulation of ARF17 for anther development and pollen formation.BMC Plant Biol. 2017 Dec 19;17(1):243. doi: 10.1186/s12870-017-1185-1. BMC Plant Biol. 2017. PMID: 29258431 Free PMC article.
-
Microsporocytic ARF17 misexpression leads to an excess callose deposition and male sterility in Arabidopsis.Plant Mol Biol. 2025 Jan 17;115(1):18. doi: 10.1007/s11103-024-01549-3. Plant Mol Biol. 2025. PMID: 39821735
-
AUXIN RESPONSE FACTOR17 is essential for pollen wall pattern formation in Arabidopsis.Plant Physiol. 2013 Jun;162(2):720-31. doi: 10.1104/pp.113.214940. Epub 2013 Apr 11. Plant Physiol. 2013. PMID: 23580594 Free PMC article.
-
The Arabidopsis CALLOSE DEFECTIVE MICROSPORE1 gene is required for male fertility through regulating callose metabolism during microsporogenesis.Plant Physiol. 2014 Apr;164(4):1893-904. doi: 10.1104/pp.113.233387. Epub 2014 Feb 24. Plant Physiol. 2014. PMID: 24567187 Free PMC article.
-
Molecular Manipulation of the miR160/AUXIN RESPONSE FACTOR Expression Module Impacts Root Development in Arabidopsis thaliana.Genes (Basel). 2024 Aug 7;15(8):1042. doi: 10.3390/genes15081042. Genes (Basel). 2024. PMID: 39202402 Free PMC article.
Cited by
-
AtC3H3, an Arabidopsis Non-TZF Gene, Enhances Salt Tolerance by Increasing the Expression of Both ABA-Dependent and -Independent Stress-Responsive Genes.Int J Mol Sci. 2024 Oct 11;25(20):10943. doi: 10.3390/ijms252010943. Int J Mol Sci. 2024. PMID: 39456724 Free PMC article.
-
The temporal regulation of TEK contributes to pollen wall exine patterning.PLoS Genet. 2020 May 14;16(5):e1008807. doi: 10.1371/journal.pgen.1008807. eCollection 2020 May. PLoS Genet. 2020. PMID: 32407354 Free PMC article.
-
Comprehensive Atlas of Wheat (Triticum aestivum L.) AUXIN RESPONSE FACTOR Expression During Male Reproductive Development and Abiotic Stress.Front Plant Sci. 2020 Sep 30;11:586144. doi: 10.3389/fpls.2020.586144. eCollection 2020. Front Plant Sci. 2020. PMID: 33101350 Free PMC article.
-
Callose synthesis during reproductive development in monocotyledonous and dicotyledonous plants.Plant Signal Behav. 2016;11(2):e1062196. doi: 10.1080/15592324.2015.1062196. Plant Signal Behav. 2016. PMID: 26451709 Free PMC article. Review.
-
Transcriptome and WGCNA reveals the potential genetic basis of photoperiod-sensitive male sterility in soybean.BMC Genomics. 2025 Feb 11;26(1):131. doi: 10.1186/s12864-025-11314-5. BMC Genomics. 2025. PMID: 39934659 Free PMC article.
References
-
- Heslop-Harrison J (1971) Wall pattern formation in angiosperm microsporogenesis. Symp Soc Exp Biol 25: 277–300. - PubMed
-
- Zinkl GM, Zwiebel BI, Grier DG, Preuss D (1999) Pollen-stigma adhesion in Arabidopsis: a species-specific interaction mediated by lipophilic molecules in the pollen exine. pp. 5431–5440. - PubMed
-
- Dickinson HG, Heslop-Harrison J (1968) Common mode of deposition for the sporopollenin of sexine and nexine. Nature 220: 926–927. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

