Examining the species-specificity of rhesus macaque cytomegalovirus (RhCMV) in cynomolgus macaques
- PMID: 25822981
- PMCID: PMC4378995
- DOI: 10.1371/journal.pone.0121339
Examining the species-specificity of rhesus macaque cytomegalovirus (RhCMV) in cynomolgus macaques
Erratum in
-
Correction: Examining the Species-Specificity of Rhesus Macaque Cytomegalovirus (RhCMV) in Cynomolgus Macaques.PLoS One. 2015 May 14;10(5):e0128733. doi: 10.1371/journal.pone.0128733. eCollection 2015. PLoS One. 2015. PMID: 25973867 Free PMC article. No abstract available.
Abstract
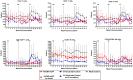
Cytomegalovirus (CMV) is a highly species-specific virus that has co-evolved with its host over millions of years and thus restricting cross-species infection. To examine the extent to which host restriction may prevent cross-species research between closely related non-human primates, we evaluated experimental infection of cynomolgus macaques with a recombinant rhesus macaque-derived CMV (RhCMV-eGFP). Twelve cynomolgus macaques were randomly allocated to three groups: one experimental group (RhCMV-eGFP) and two control groups (UV-inactivated RhCMV-eGFP or media alone). The animals were given two subcutaneous inoculations at week 0 and week 8, and a subset of animals received an intravenous inoculation at week 23. No overt clinical or haematological changes were observed and PBMCs isolated from RhCMV-eGFP inoculated animals had comparable eGFP- and IE-1-specific cellular responses to the control animals. Following inoculation with RhCMV-eGFP, we were unable to detect evidence of infection in any blood or tissue samples up to 4 years post-inoculation, using sensitive viral co-culture, qPCR, and Western blot assays. Co-culture of urine and saliva samples demonstrated the presence of endogenous cynomolgus CMV (CyCMV) cytopathic effect, however no concomitant eGFP expression was observed. The absence of detectable RhCMV-eGFP suggests that the CyCMV-seropositive cynomolgus macaques were not productively infected with RhCMV-eGFP under these inoculation conditions. In a continued effort to develop CMV as a viral vector for an HIV/SIV vaccine, these studies demonstrate that CMV is highly restricted to its host species and can be highly affected by laboratory cell culture. Consideration of the differences between lab-adapted and primary viruses with respect to species range and cell tropism should be a priority in evaluating CMV as vaccine vector for HIV or other pathogens at the preclinical development stage.
Conflict of interest statement
Figures




References
-
- McGeoch DJ, Cook S, Dolan A, Jamieson FE, Telford EA (1995) Molecular phylogeny and evolutionary timescale for the family of mammalian herpesviruses. Journal of molecular biology 247: 443–458. - PubMed
-
- Dunn HS, Haney DJ, Ghanekar SA, Stepick-Biek P, Lewis DB, et al. (2002) Dynamics of CD4 and CD8 T cell responses to cytomegalovirus in healthy human donors. The Journal of infectious diseases 186: 15–22. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

