Coiled coil rich proteins (Ccrp) influence molecular pathogenicity of Helicobacter pylori
- PMID: 25822999
- PMCID: PMC4379086
- DOI: 10.1371/journal.pone.0121463
Coiled coil rich proteins (Ccrp) influence molecular pathogenicity of Helicobacter pylori
Abstract
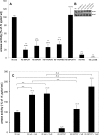
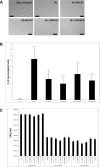
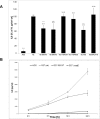
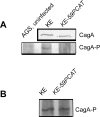
Pathogenicity of the human pathogen Helicobacter pylori relies on its capacity to adapt to a hostile environment and to escape the host response. Although there have been great advances in our understanding of the bacterial cytoskeleton, major gaps remain in our knowledge of its contribution to virulence. In this study we have explored the influence of coiled coil rich proteins (Ccrp) cytoskeletal elements on pathogenicity factors of H. pylori. Deletion of any of the ccrp resulted in a strongly decreased activity of the main pathogenicity factor urease. We further investigated their role using in vitro co-culture experiments with the human gastric adenocarcinoma cell line AGS modeling H. pylori - host cell interactions. Intriguingly, host cell showed only a weak "scattering/hummingbird" phenotype, in which host cells are transformed from a uniform polygonal shape into a severely elongated state characterized by the formation of needle-like projections, after co-incubation with any ccrp deletion mutant. Furthermore, co-incubation with the ccrp59 mutant resulted in reduced type IV secretion system associated activities, e.g. IL-8 production and CagA translocation/phosphorylation. Thus, in addition to their role in maintaining the helical cell shape of H. pylori Ccrp proteins influence many cellular processes and are thereby crucial for the virulence of this human pathogen.
Conflict of interest statement
Figures

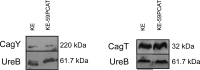
References
-
- Marshall BJ, Warren JR (1984) Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet 1: 1311–1315. - PubMed
-
- Blaser MJ (1990) Helicobacter pylori and the pathogenesis of gastroduodenal inflammation. JInfectDis 161: 626–633. - PubMed
-
- (1993) An international association between Helicobacter pylori infection and gastric cancer. The EUROGAST Study Group. Lancet 341: 1359–1362. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

