Leukocyte inclusion within a platelet rich plasma-derived fibrin scaffold stimulates a more pro-inflammatory environment and alters fibrin properties
- PMID: 25823008
- PMCID: PMC4379078
- DOI: 10.1371/journal.pone.0121713
Leukocyte inclusion within a platelet rich plasma-derived fibrin scaffold stimulates a more pro-inflammatory environment and alters fibrin properties
Abstract
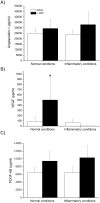
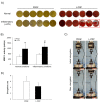
One of the main differences among platelet-rich plasma (PRP) products is the inclusion of leukocytes that may affect the biological efficacy of these autologous preparations. The purpose of this study was to evaluate whether the addition of leukocytes modified the morphological, biomechanical and biological properties of PRP under normal and inflammatory conditions. The release of pro-inflammatory cytokines from plasma rich in growth factors (PRGF) and leukocyte-platelet rich plasma (L-PRP) scaffolds was determined by enzyme-linked immunosorbent assay (ELISA) and was significantly increased under an inflammatory condition when leukocytes were included in the PRP. Fibroblasts and osteoblasts treated with L-PRP, under an inflammatory situation, underwent a greater activation of NFĸB pathway, proliferated significantly less and secreted a higher concentration of pro-inflammatory cytokines. These cellular events were assessed through Western blot and fluorimetric and ELISA methods, respectively. Therefore, the inclusion of leukocytes induced significantly higher pro-inflammatory conditions.
Conflict of interest statement
Figures






Similar articles
-
Morphogen and proinflammatory cytokine release kinetics from PRGF-Endoret fibrin scaffolds: evaluation of the effect of leukocyte inclusion.J Biomed Mater Res A. 2015 Mar;103(3):1011-20. doi: 10.1002/jbm.a.35244. Epub 2014 Jun 12. J Biomed Mater Res A. 2015. PMID: 24890049
-
Advantages of pure platelet-rich plasma compared with leukocyte- and platelet-rich plasma in promoting repair of bone defects.J Transl Med. 2016 Mar 15;14:73. doi: 10.1186/s12967-016-0825-9. J Transl Med. 2016. PMID: 26980293 Free PMC article.
-
Cellular composition modifies the biological properties and stability of platelet rich plasma membranes for tissue engineering.J Biomed Mater Res A. 2023 Nov;111(11):1710-1721. doi: 10.1002/jbm.a.37579. Epub 2023 Jun 15. J Biomed Mater Res A. 2023. PMID: 37318048
-
Do the fibrin architecture and leukocyte content influence the growth factor release of platelet concentrates? An evidence-based answer comparing a pure platelet-rich plasma (P-PRP) gel and a leukocyte- and platelet-rich fibrin (L-PRF).Curr Pharm Biotechnol. 2012 Jun;13(7):1145-52. doi: 10.2174/138920112800624382. Curr Pharm Biotechnol. 2012. PMID: 21740377 Review.
-
Microbicidal properties of Leukocyte- and Platelet-Rich Plasma/Fibrin (L-PRP/L-PRF): new perspectives.J Biol Regul Homeost Agents. 2012 Apr-Jun;26(2 Suppl 1):43S-52S. J Biol Regul Homeost Agents. 2012. PMID: 23648198 Review.
Cited by
-
Effect of Leukocyte-Rich and Platelet-Rich Plasma on Healing of a Horizontal Medial Meniscus Tear in a Rabbit Model.Biomed Res Int. 2015;2015:179756. doi: 10.1155/2015/179756. Epub 2015 Jun 9. Biomed Res Int. 2015. PMID: 26180783 Free PMC article.
-
An Autologous Topical Serum Derived from Platelet-Rich Plasma Therapy for the Management of Sensitive Skin Alterations: A Case Series Report.Clin Cosmet Investig Dermatol. 2022 Sep 29;15:2077-2086. doi: 10.2147/CCID.S379323. eCollection 2022. Clin Cosmet Investig Dermatol. 2022. PMID: 36199385 Free PMC article.
-
The Use of Autologous Protein Solution (Pro-Stride®) and Leukocyte-Rich Platelet-Rich Plasma (Restigen®) in Canine Medicine.Vet Med (Auckl). 2021 Mar 19;12:53-65. doi: 10.2147/VMRR.S286913. eCollection 2021. Vet Med (Auckl). 2021. PMID: 33777723 Free PMC article.
-
Platelet-Rich Plasma Does Not Inhibit Inflammation or Promote Regeneration in Human Osteoarthritic Chondrocytes In Vitro Despite Increased Proliferation.Cartilage. 2021 Dec;13(2_suppl):991S-1003S. doi: 10.1177/1947603520961162. Epub 2020 Sep 24. Cartilage. 2021. PMID: 32969277 Free PMC article.
-
Effect of the use of platelet concentrates on new bone formation in alveolar ridge preservation: a systematic review, meta-analysis, and trial sequential analysis.Clin Oral Investig. 2023 Aug;27(8):4131-4146. doi: 10.1007/s00784-023-05126-8. Epub 2023 Jul 13. Clin Oral Investig. 2023. PMID: 37439800 Free PMC article.
References
-
- Humes HD (2005) Translational medicine and the National Institutes of Health road map: steep grades and tortuous curves. J Lab Clin Med 146: 51–54. - PubMed
-
- Crovetti G, Martinelli G, Issi M, Barone M, Guizzardi M, Campanati B, et al. (2004) Platelet gel for healing cutaneous chronic wounds. Transfus Apher Sci 30: 145–151. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

