Silencing the double-stranded RNA binding protein DGCR8 inhibits ovarian cancer cell proliferation, migration, and invasion
- PMID: 25823356
- PMCID: PMC3984613
- DOI: 10.1007/s11095-013-1219-9
Silencing the double-stranded RNA binding protein DGCR8 inhibits ovarian cancer cell proliferation, migration, and invasion
Abstract
Purpose: To evaluate the role of DiGeorge Critical Region 8 (DGCR8), a key component of miRNA biogenesis pathway in ovarian cancer.
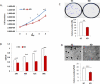
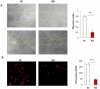
Methods: The expression of DGCR8 in ovarian cancer was detected by immunostaining and DGCR8 knockdown in ovarian cancer cells was achieved using lentiviral shRNA. Differential expression of miRNAs was determined using Nanostring miRNA arrays and validated by real-time RT-PCR.
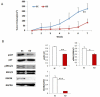
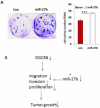
Results: DGCR8 was highly expressed in ovarian cancer. Knockdown of DGCR8 expression inhibits cell proliferation, migration, and invasion, as well as sensitizes cells to apoptosis induced by the chemotherapeutic drug cisplatin. Cellular survival pathways including ERK1/2 mitogen-activated protein kinase and phosphatidylinositol 3-kinase/AKT were attenuated in DGCR8 knockdown cells. DGCR8 knockdown results in dysregulated miRNA gene expression. miR-27b was identified as the most highly down-regulated miRNA in DGCR8 knockdown cells and promoted cell proliferation in ovarian cancer cells.
Conclusions: DGCR8 functions as an oncogene in ovarian cancer, which is in part mediated by miR-27b.
Figures



References
-
- Gregory RI, Yan KP, Amuthan G, Chendrimada T, Doratotaj B, Cooch N, Shiekhattar R. Nature. 2004;432:235–240. - PubMed
-
- Landthaler M, Yalcin A, Tuschl T. Curr Biol. 2004;14:2162–2167. - PubMed
-
- Han L, Zhang A, Zhou X, Xu P, Wang GX, Pu PY, Kang CS. Int J Oncol. 2010;37:299–305. - PubMed
-
- Sand M, Gambichler T, Skrygan M, Sand D, Scola N, Altmeyer P, Bechara FG. Cancer Invest. 2010;28:649–653. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

