Does Caspase-6 Have a Role in Perinatal Brain Injury?
- PMID: 25823427
- PMCID: PMC4876595
- DOI: 10.1159/000375368
Does Caspase-6 Have a Role in Perinatal Brain Injury?
Abstract
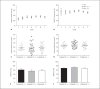
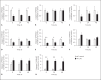
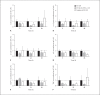
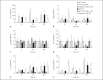
Apoptotic mechanisms are centre stage for the development of injury in the immature brain, and caspases have been shown to play a pivotal role during brain development and in response to injury. The inhibition of caspases using broad-spectrum agents such as Q-VD-OPh is neuroprotective in the immature brain. Caspase-6, an effector caspase, has been widely researched in neurodevelopmental disorders and found to be important following adult stroke, but its function in the neonatal brain has yet to be detailed. Furthermore, caspases may be important in microglial activation; microglia are required for optimal brain development and following injury, and their close involvement during neuronal cell death suggests that apoptotic cues such as caspase activation may be important in microglial activation. Therefore, in this study we aimed to investigate the possible apoptotic and non-apoptotic functions caspase-6 may have in the immature brain in response to hypoxia-ischaemia. We examined whether caspases are involved in microglial activation. We assessed cleaved caspase-6 expression following hypoxia-ischaemia and conducted primary microglial cultures to assess whether the broad-spectrum inhibitor Q-VD-OPh or caspase-6 gene deletion affected lipopolysaccharide (LPS)-mediated microglial activation and phenotype. We observed cleaved caspase-6 expression to be low but present in the cell body and cell processes in both a human case of white matter injury and 72 h following hypoxia-ischaemia in the rat. Gene deletion of caspase-6 did not affect the outcome of brain injury following mild (50 min) or severe (60 min) hypoxia-ischaemia. Interestingly, we did note that cleaved caspase-6 was co-localised with microglia that were not of apoptotic morphology. We observed that mRNA of a number of caspases was modulated by low-dose LPS stimulation of primary microglia. Q-VD-OPh treatment and caspase-6 gene deletion did not affect microglial activation but modified slightly the M2b phenotype response by changing the time course of SOCS3 expression after LPS administration. Our results suggest that the impact of active caspase-6 in the developing brain is subtle, and we believe there are predominantly other caspases (caspase-2, -3, -8, -9) that are essential for the cell death processes in the immature brain.
© 2015 S. Karger AG, Basel.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

