Practice guidelines for sedation and analgesia management of critically ill children: a pilot study evaluating guideline impact and feasibility in the PICU
- PMID: 25823444
- PMCID: PMC4386214
- DOI: 10.1136/bmjopen-2014-006428
Practice guidelines for sedation and analgesia management of critically ill children: a pilot study evaluating guideline impact and feasibility in the PICU
Abstract
Aims: The aim of this study was to develop and implement guidelines for sedation and analgesia management in the paediatric intensive care unit (PICU) and evaluate the impact, feasibility and acceptability of these as part of a programme of research in this area and as a prelude to future trial work.
Method: This pilot study used a pre-post design using a historical control.
Setting: Two PICUs at different hospitals in an Australian metropolitan city.
Participants: Patients admitted to the PICU and ventilated for ≥24 h, aged more than 1 month and not admitted for seizure management or terminal care.
Intervention: Guidelines for sedation and analgesia management for critically ill children including algorithm and assessment tools.
Outcome variables: In addition to key outcome variables (ventilation time, medication dose and duration, length of stay), feasibility outcomes data (recruitment, data collection, safety) were evaluated. Guideline adherence was assessed through chart audit and staff were surveyed about merit and the use of guidelines.
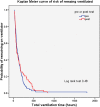
Results: The guidelines were trialled for a total of 12 months on 63 patients and variables compared with the historical control group (n=75). Analysis revealed differences in median Morphine infusion duration between groups (pretest 3.63 days (87 h) vs post-test 2.83 days (68 h), p=0.05) and maximum doses (pretest 120 μg/kg/h vs post-test 97.5 μg/kg/h) with no apparent change to ventilation duration. Chart audit revealed varied use of tools, but staff were positive about the guidelines and their use in practice.
Conclusions: The sedation guidelines impacted on the duration and dosage of agents without any apparent impact on ventilation duration or length of stay. Furthermore, the guidelines appeared to be feasible and acceptable in clinical practice. The results of the study have laid the foundation for follow-up studies in withdrawal from sedation, point prevalence and longitudinal studies of sedation practices as well as drug trial work.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://group.bmj.com/group/rights-licensing/permissions.
Figures
References
-
- Bavdekar SB, Mahajan MD, Chandu KV. Analgesia and sedation in paediatric intensive care unit. J Postgrad Med 1999;45:95–102. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources