Initial Results of Image-Guided Percutaneous Ablation as Second-Line Treatment for Symptomatic Vascular Anomalies
- PMID: 25823573
- PMCID: PMC4567898
- DOI: 10.1007/s00270-015-1079-2
Initial Results of Image-Guided Percutaneous Ablation as Second-Line Treatment for Symptomatic Vascular Anomalies
Abstract
Purpose: The purpose of this study was to determine the feasibility, safety, and early effectiveness of percutaneous image-guided ablation as second-line treatment for symptomatic soft-tissue vascular anomalies (VA).
Materials and methods: An IRB-approved retrospective review was undertaken of all patients who underwent percutaneous image-guided ablation as second-line therapy for treatment of symptomatic soft-tissue VA during the period from 1/1/2008 to 5/20/2014. US/CT- or MRI-guided and monitored cryoablation or MRI-guided and monitored laser ablation was performed. Clinical follow-up began at one-month post-ablation.
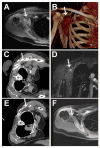
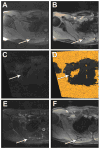
Results: Eight patients with nine torso or lower extremity VA were treated with US/CT (N = 4) or MRI-guided (N = 2) cryoablation or MRI-guided laser ablation (N = 5) for moderate to severe pain (N = 7) or diffuse bleeding secondary to hemangioma-thrombocytopenia syndrome (N = 1). The median maximal diameter was 9.0 cm (6.5-11.1 cm) and 2.5 cm (2.3-5.3 cm) for VA undergoing cryoablation and laser ablation, respectively. Seven VA were ablated in one session, one VA initially treated with MRI-guided cryoablation for severe pain was re-treated with MRI-guided laser ablation due to persistent moderate pain, and one VA was treated in a planned two-stage session due to large VA size. At an average follow-up of 19.8 months (range 2-62 months), 7 of 7 patients with painful VA reported symptomatic pain relief. There was no recurrence of bleeding at five-year post-ablation in the patient with hemangioma-thrombocytopenia syndrome. There were two minor complications and no major complications.
Conclusion: Image-guided percutaneous ablation is a feasible, safe, and effective second-line treatment option for symptomatic VA.
Keywords: CT; Cryoablation; Laser ablation; MRI; Vascular anomaly; Vascular malformation; Vasoproliferative neoplasm.
Conflict of interest statement
Matthew R. Callstrom has received research grants from Thermedical Inc., General Electric Company, Siemens AG and Galil Medical Ltd. Scott Thompson, Michael A. McKusick and David A. Woodrum report no conflicts of interest related to the subject of this manuscript.
Figures

Similar articles
-
Percutaneous MR Imaging-Guided Laser Ablation and Cryoablation for the Treatment of Pediatric and Adult Symptomatic Peripheral Soft Tissue Vascular Anomalies.J Vasc Interv Radiol. 2021 Oct;32(10):1417-1424. doi: 10.1016/j.jvir.2021.07.019. Epub 2021 Jul 29. J Vasc Interv Radiol. 2021. PMID: 34332090
-
Percutaneous image-guided cryoablation of venous malformation and fibro-adipose vascular anomaly: prognostic factors of clinical efficacy.Eur Radiol. 2025 Oct;35(10):6554-6563. doi: 10.1007/s00330-025-11545-w. Epub 2025 Apr 5. Eur Radiol. 2025. PMID: 40185927
-
Percutaneous MR Imaging-Guided Laser Ablation for the Treatment of Symptomatic Cervicofacial Vascular Malformations.J Vasc Interv Radiol. 2023 Feb;34(2):197-204. doi: 10.1016/j.jvir.2022.10.019. Epub 2022 Oct 17. J Vasc Interv Radiol. 2023. PMID: 36257582
-
Percutaneous cryoablation of hepatic tumors: long-term experience of a large U.S. series.Abdom Radiol (NY). 2016 Apr;41(4):767-80. doi: 10.1007/s00261-016-0687-x. Abdom Radiol (NY). 2016. PMID: 26960728 Review.
-
An overview of interventional radiology techniques for the diagnosis and management of vascular anomalies: Part 1.Pediatr Dermatol. 2023 Mar;40(2):242-249. doi: 10.1111/pde.15246. Epub 2023 Jan 9. Pediatr Dermatol. 2023. PMID: 36623539 Review.
Cited by
-
Epithelioid hemangioma of the scapula treated with chemoembolization and microwave ablation: Α case report.Acta Orthop Traumatol Turc. 2018 Mar;52(2):157-161. doi: 10.1016/j.aott.2017.01.003. Epub 2017 Jan 31. Acta Orthop Traumatol Turc. 2018. PMID: 28159479 Free PMC article.
-
Percutaneous cryoablation of symptomatic venous malformations as a second-line therapeutic option: a five-year single institution experience.Eur Radiol. 2017 Dec;27(12):5015-5023. doi: 10.1007/s00330-017-4892-y. Epub 2017 Jul 4. Eur Radiol. 2017. PMID: 28677056
-
Resolution of Pain after Percutaneous Image-Guided Cryoablation of Extraperitoneal Endometriosis.J Vasc Interv Radiol. 2023 Jul;34(7):1192-1198. doi: 10.1016/j.jvir.2023.03.025. Epub 2023 Mar 30. J Vasc Interv Radiol. 2023. PMID: 37003579 Free PMC article.
-
Image-Guided Percutaneous Ablation for Primary and Metastatic Tumors.Diagnostics (Basel). 2022 May 24;12(6):1300. doi: 10.3390/diagnostics12061300. Diagnostics (Basel). 2022. PMID: 35741109 Free PMC article. Review.
-
Non-invasive magnetic resonance-guided high intensity focused ultrasound ablation of a vascular malformation in the lower extremity: a case report.J Ther Ultrasound. 2015 Dec 30;3:23. doi: 10.1186/s40349-015-0042-7. eCollection 2015. J Ther Ultrasound. 2015. PMID: 26719802 Free PMC article.
References
-
- Lowe LH, Marchant TC, Rivard DC, Scherbel AJ. Vascular malformations: classification and terminology the radiologist needs to know. Semin Roentgenol. 2012;47(2):106–117. - PubMed
-
- Kelly M. Kasabach-Merritt phenomenon. Pediatr Clin North Am. 2010;57(5):1085–1089. - PubMed
-
- van der Vleuten CJ, Kater A, Wijnen MH, Schultze Kool LJ, Rovers MM. Effectiveness of sclerotherapy, surgery, and laser therapy in patients with venous malformations: a systematic review. Cardiovasc Intervent Radiol. 2014;37(4):977–989. - PubMed
-
- Kim AH, Ko HK, Won JY, Lee do Y. Percutaneous radiofrequency ablation: a novel treatment of facial venous malformation. J Vasc Surg. 2009;50(2):424–427. - PubMed
-
- Childs DD, Emory CL. Successful treatment of intramuscular venous malformation with image-guided radiofrequency ablation. J Vasc Interv Radiol. 2012;23(10):1391–1393. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

