Modeling and Simulation to Support Phase 2 Dose Selection for RG7652, a Fully Human Monoclonal Antibody Against Proprotein Convertase Subtilisin/Kexin Type 9
- PMID: 25823668
- PMCID: PMC4476990
- DOI: 10.1208/s12248-015-9750-8
Modeling and Simulation to Support Phase 2 Dose Selection for RG7652, a Fully Human Monoclonal Antibody Against Proprotein Convertase Subtilisin/Kexin Type 9
Abstract
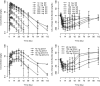
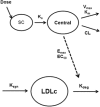
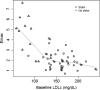
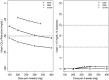
RG7652 is a fully humanized monoclonal antibody targeting human PCSK9, a regulator of serum low density lipoprotein cholesterol (LDLc) levels. RG7652 prevents degradation of the hepatic LDLc receptors by blocking PCSK9 binding and thereby resulting in efficient LDLc uptake by hepatocytes. The pharmacokinetics of RG7652 have been evaluated in healthy subjects after single and multiple subcutaneous doses. Pharmacokinetic (PK) and pharmacodynamic (PD) models were developed to explain the antibody PK and LDLc time course data. The PK and PD models based on data from healthy subjects were used to simulate the effects of RG7652 on LDLc levels for a range of potential dose regimens in patients with coronary heart disease. A one-compartment PK model combined with an indirect PD response model was able to adequately describe the PK and LDLc data. Simulations of 400 mg every 4 weeks or 800 mg every 8 weeks regimens show significant LDLc reduction and suggest that dosing RG7652 once every month or once every 2 months is predicted to be optimal for the treatment of hypercholesterolemia. The PK and PD model successfully described the PK and LDLc data from healthy subjects in a Phase 1 study, and the model-based simulations provided useful insights and quantitative understanding for the selection of Phase 2 study doses in patients with coronary heart disease. The approach used in the case study demonstrates the utility of modeling and simulation in designing dose-ranging studies.
Figures
Similar articles
-
A phase 1 study to evaluate the safety and LDL cholesterol-lowering effects of RG7652, a fully human monoclonal antibody against proprotein convertase subtilisin/kexin type 9.Clin Cardiol. 2017 Jul;40(7):503-511. doi: 10.1002/clc.22687. Epub 2017 Mar 22. Clin Cardiol. 2017. PMID: 28326559 Free PMC article. Clinical Trial.
-
Population Pharmacokinetics (PK) and Pharmacodynamics (PD) Analysis of LY3015014, a Monoclonal Antibody to Protein Convertase Subtilisin/Kexin Type 9 (PCSK9) in Healthy Subjects and Hypercholesterolemia Patients.Pharm Res. 2017 Jan;34(1):185-192. doi: 10.1007/s11095-016-2054-6. Epub 2016 Nov 7. Pharm Res. 2017. PMID: 27822850 Clinical Trial.
-
Critical role of bioanalytical strategies in investigation of clinical PK observations, a Phase I case study.MAbs. 2014;6(6):1500-8. doi: 10.4161/mabs.36208. MAbs. 2014. PMID: 25484037 Free PMC article. Clinical Trial.
-
Proprotein convertase subtilisin kexin 9 (PCSK9) inhibitors in the treatment of hypercholesterolemia and other pathologies.Curr Pharm Des. 2013;19(17):3161-72. doi: 10.2174/13816128113199990313. Curr Pharm Des. 2013. PMID: 23317404 Review.
-
Potential of proprotein convertase subtilisin/kexin type 9 based therapeutics.Curr Atheroscler Rep. 2013 Mar;15(3):310. doi: 10.1007/s11883-013-0310-3. Curr Atheroscler Rep. 2013. PMID: 23371064 Review.
Cited by
-
PCSK9 inhibitors: A new era of lipid lowering therapy.World J Cardiol. 2017 Feb 26;9(2):76-91. doi: 10.4330/wjc.v9.i2.76. World J Cardiol. 2017. PMID: 28289523 Free PMC article. Review.
-
Modeling and Simulation to Support Phase Ib/IIa Dose Selection for WBP216, A Long Half-Life Fully Human Monoclonal Antibody Against Interleukin-6.Front Pharmacol. 2021 Feb 18;12:617265. doi: 10.3389/fphar.2021.617265. eCollection 2021. Front Pharmacol. 2021. PMID: 33679400 Free PMC article.
-
From PK/PD to QSP: Understanding the Dynamic Effect of Cholesterol-Lowering Drugs on Atherosclerosis Progression and Stratified Medicine.Curr Pharm Des. 2016;22(46):6903-6910. doi: 10.2174/1381612822666160905095402. Curr Pharm Des. 2016. PMID: 27592718 Free PMC article. Review.
-
PCSK9 Monoclonal Antibodies: An Overview.Heart Views. 2020 Apr-Jun;21(2):97-103. doi: 10.4103/HEARTVIEWS.HEARTVIEWS_20_20. Epub 2020 Jun 29. Heart Views. 2020. PMID: 33014302 Free PMC article.
-
Quantitative characterization of the mechanism of action and impact of a 'proteolysis-permitting' anti-PCSK9 antibody.MAbs. 2017 Feb/Mar;9(2):285-296. doi: 10.1080/19420862.2016.1270490. MAbs. 2017. PMID: 27981884 Free PMC article.
References
-
- Third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) final report. Circulation. 2002;106(25):3143-421. - PubMed
-
- Sniderman A, Thanassoulis G, Couture P, Williams K, Alam A, Furberg CD. Is lower and lower better and better? A re-evaluation of the evidence from the Cholesterol Treatment Trialists’ Collaboration meta-analysis for low-density lipoprotein lowering. J Clin Lipidol. 2012;6(4):303–9. doi: 10.1016/j.jacl.2012.05.004. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

