Triaminopyrimidine is a fast-killing and long-acting antimalarial clinical candidate
- PMID: 25823686
- PMCID: PMC4389225
- DOI: 10.1038/ncomms7715
Triaminopyrimidine is a fast-killing and long-acting antimalarial clinical candidate
Abstract
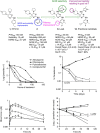
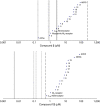
The widespread emergence of Plasmodium falciparum (Pf) strains resistant to frontline agents has fuelled the search for fast-acting agents with novel mechanism of action. Here, we report the discovery and optimization of novel antimalarial compounds, the triaminopyrimidines (TAPs), which emerged from a phenotypic screen against the blood stages of Pf. The clinical candidate (compound 12) is efficacious in a mouse model of Pf malaria with an ED99 <30 mg kg(-1) and displays good in vivo safety margins in guinea pigs and rats. With a predicted half-life of 36 h in humans, a single dose of 260 mg might be sufficient to maintain therapeutic blood concentration for 4-5 days. Whole-genome sequencing of resistant mutants implicates the vacuolar ATP synthase as a genetic determinant of resistance to TAPs. Our studies highlight the potential of TAPs for single-dose treatment of Pf malaria in combination with other agents in clinical development.
Figures

References
-
- WHO. World Malaria Report 2014, http://www.who.int/malaria/publications/world_malaria_report_2014/en/.
-
- White N. J. et al. Malaria. Lancet 383, 723–735 (2014) . - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

