Dwelling quietly in the rich club: brain network determinants of slow cortical fluctuations
- PMID: 25823864
- PMCID: PMC4387508
- DOI: 10.1098/rstb.2014.0165
Dwelling quietly in the rich club: brain network determinants of slow cortical fluctuations
Abstract
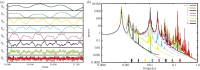
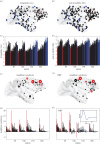
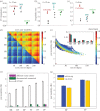
For more than a century, cerebral cartography has been driven by investigations of structural and morphological properties of the brain across spatial scales and the temporal/functional phenomena that emerge from these underlying features. The next era of brain mapping will be driven by studies that consider both of these components of brain organization simultaneously--elucidating their interactions and dependencies. Using this guiding principle, we explored the origin of slowly fluctuating patterns of synchronization within the topological core of brain regions known as the rich club, implicated in the regulation of mood and introspection. We find that a constellation of densely interconnected regions that constitute the rich club (including the anterior insula, amygdala and precuneus) play a central role in promoting a stable, dynamical core of spontaneous activity in the primate cortex. The slow timescales are well matched to the regulation of internal visceral states, corresponding to the somatic correlates of mood and anxiety. In contrast, the topology of the surrounding 'feeder' cortical regions shows unstable, rapidly fluctuating dynamics likely to be crucial for fast perceptual processes. We discuss these findings in relation to psychiatric disorders and the future of connectomics.
Keywords: brain dynamics; chronoarchitecture; connectome; core-periphery axis; metastability; prefrontal hierarchy.
© 2015 The Author(s) Published by the Royal Society. All rights reserved.
Figures



References
-
- Brodmann K. 1909. Vergleichende Lokalisationslehre der Grosshirnrinde in ihren Prinzipien dargestellt auf Grund des Zellenbaues: Barth.
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

