Characterizing opioid withdrawal during double-blind buprenorphine detoxification
- PMID: 25823907
- PMCID: PMC4447545
- DOI: 10.1016/j.drugalcdep.2015.02.033
Characterizing opioid withdrawal during double-blind buprenorphine detoxification
Abstract
Background: Prescription opioid (PO) abuse has become an urgent public health issue in the United States. Detoxification is one important treatment option, yet relatively little is known about the time course and severity of opioid withdrawal during buprenorphine detoxification.
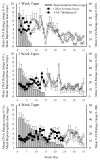
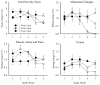
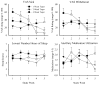
Methods: This is a secondary analysis of data from a randomized, placebo-controlled, double-blind evaluation of 1, 2, and 4-week outpatient buprenorphine tapers among primary prescription opioid (PO) abusers. The aim is to characterize the time course and severity of buprenorphine withdrawal under rigorous, double-blind conditions, across multiple taper durations, and using multiple withdrawal-related measures (i.e., self-report and observer ratings, pupil diameter, ancillary medication utilization). Participants were PO-dependent adults undergoing buprenorphine detoxification and biochemically-verified to be continuously abstinent from opioids during their taper (N = 28).
Results: Participants randomly assigned to the 4-week taper regimen experienced a relatively mild and stable course of withdrawal, with few peaks in severity. In contrast, the 1- and 2-week taper groups experienced stark increases in withdrawal severity during the week following the last buprenorphine dose, followed by declines in withdrawal severity thereafter. The 4-week taper group also reported significantly fewer disruptions in sleep compared to the other experimental groups. When predictors of withdrawal were examined, baseline ratings of "Expected Withdrawal Severity" was the most robust predictor of withdrawal experienced during the taper.
Conclusion: Data from this trial may inform clinicians about the expected time course, magnitude, and pattern of buprenorphine withdrawal and aid efforts to identify patients who may need additional clinical support during outpatient buprenorphine detoxification.
Keywords: Buprenorphine; Detoxification; Prescription opioid; Taper; Withdrawal.
Copyright © 2015 Elsevier Ireland Ltd. All rights reserved.
Conflict of interest statement
Conflict of Interest: No authors have a conflict of interest to report.
Figures
References
-
- Amass L, Bickel WK, Higgins ST, Hughes JR. A preliminary investigation of outcome following gradual or rapid buprenorphine detoxification. J Addict Dis. 1994;13:33–45. - PubMed
-
- Appel PW, Ellison AA, Jansky HK, Oldak R. Barriers to enrollment in drug abuse treatment and suggestions for reducing them: opinions of drug injecting street outreach clients and other system stakeholders. Am J Drug Alcohol Abuse. 2004;30:129–153. - PubMed
-
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. - PubMed
-
- Becker AB, Strain EC, Bigelow GE, Stitzer ML, Johnson RE. Gradual dose taper following chronic buprenorphine. Am J Addict. 2001;10:111–121. - PubMed
-
- Best D, Gossop M, Stewart D, Marsden J, Lehmann P, Strang J. Continued heroin use during methadone treatment: relationships between frequency of use and reasons reported for heroin use. Drug Alcohol Depend. 1999;53:191–195. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

