Activated protein C: biased for translation
- PMID: 25824691
- PMCID: PMC4424414
- DOI: 10.1182/blood-2015-02-355974
Activated protein C: biased for translation
Abstract
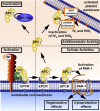
The homeostatic blood protease, activated protein C (APC), can function as (1) an antithrombotic on the basis of inactivation of clotting factors Va and VIIIa; (2) a cytoprotective on the basis of endothelial barrier stabilization and anti-inflammatory and antiapoptotic actions; and (3) a regenerative on the basis of stimulation of neurogenesis, angiogenesis, and wound healing. Pharmacologic therapies using recombinant human and murine APCs indicate that APC provides effective acute or chronic therapies for a strikingly diverse range of preclinical injury models. APC reduces the damage caused by the following: ischemia/reperfusion in brain, heart, and kidney; pulmonary, kidney, and gastrointestinal inflammation; sepsis; Ebola virus; diabetes; and total lethal body radiation. For these beneficial effects, APC alters cell signaling networks and gene expression profiles by activating protease-activated receptors 1 and 3. APC's activation of these G protein-coupled receptors differs completely from thrombin's activation mechanism due to biased signaling via either G proteins or β-arrestin-2. To reduce APC-associated bleeding risk, APC variants were engineered to lack >90% anticoagulant activity but retain normal cell signaling. Such a neuroprotective variant, 3K3A-APC (Lys191-193Ala), has advanced to clinical trials for ischemic stroke. A rich data set of preclinical knowledge provides a solid foundation for potential translation of APC variants to future novel therapies.
© 2015 by The American Society of Hematology.
Figures




References
-
- Mosnier LO, Zlokovic BV, Griffin JH. The cytoprotective protein C pathway. Blood. 2007;109(8):3161–3172. - PubMed
-
- Dömötör E, Benzakour O, Griffin JH, Yule D, Fukudome K, Zlokovic BV. Activated protein C alters cytosolic calcium flux in human brain endothelium via binding to endothelial protein C receptor and activation of protease activated receptor-1. Blood. 2003;101(12):4797–4801. - PubMed
-
- Griffin JH, Zlokovic B, Fernández JA. Activated protein C: potential therapy for severe sepsis, thrombosis, and stroke. Semin Hematol. 2002;39(3):197–205. - PubMed
-
- Gupta A, Williams MD, Macias WL, Molitoris BA, Grinnell BW. Activated protein C and acute kidney injury: Selective targeting of PAR-1. Curr Drug Targets. 2009;10(12):1212–1226. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

