Analysis of the effects of cigarette smoke on staphylococcal virulence phenotypes
- PMID: 25824841
- PMCID: PMC4432760
- DOI: 10.1128/IAI.00303-15
Analysis of the effects of cigarette smoke on staphylococcal virulence phenotypes
Abstract
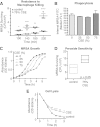
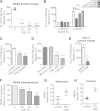
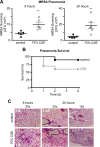
Cigarette smoking is the leading preventable cause of death, disease, and disability worldwide. It is well established that cigarette smoke provokes inflammatory activation and impairs antimicrobial functions of human immune cells. Here we explore whether cigarette smoke likewise affects the virulence properties of an important human pathogen, Staphylococcus aureus, and in particular methicillin-resistant S. aureus (MRSA), one of the leading causes of invasive bacterial infections. MRSA colonizes the nasopharynx and is thus exposed to inhalants, including cigarette smoke. MRSA exposed to cigarette smoke extract (CSE-MRSA) was more resistant to macrophage killing (4-fold higher survival; P < 0.0001). CSE-MRSA demonstrated reduced susceptibility to cell lysis (1.78-fold; P = 0.032) and antimicrobial peptide (AMP) (LL-37) killing (MIC, 8 μM versus 4 μM). CSE modified the surface charge of MRSA in a dose-dependent fashion, impairing the binding of particles with charge similar to that of AMPs by 90% (P < 0.0001). These changes persisted for 24 h postexposure, suggesting heritable modifications. CSE exposure increased hydrophobicity by 55% (P < 0.0001), which complemented findings of increased MRSA adherence and invasion of epithelial cells. CSE induced upregulation of mprF, consistent with increased MRSA AMP resistance. S. aureus without mprF had no change in surface charge upon exposure to CSE. In vivo, CSE-MRSA pneumonia induced higher mouse mortality (40% versus 10%) and increased bacterial burden at 8 and 20 h postinfection compared to control MRSA-infected mice (P < 0.01). We conclude that cigarette smoke-induced immune resistance phenotypes in MRSA may be an additional factor contributing to susceptibility to infectious disease in cigarette smokers.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures

References
-
- Anonymous. 2014. The health consequences of smoking—50 years of progress: a report of the surgeon general. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, Atlanta, GA. - PubMed
-
- WHO. 2011. Report on the global tobacco epidemic. World Health Organization, Geneva, Switzerland.
-
- CDC. 2012. Emerging Infections Program Network. Methicillin-resistant Staphylococcus aureus. Active Bacterial Core Surveillance (ABC) report. Centers for Disease Control and Prevention, Atlanta, GA.

