Novel familial dilated cardiomyopathy mutation in MYL2 affects the structure and function of myosin regulatory light chain
- PMID: 25825243
- PMCID: PMC4472530
- DOI: 10.1111/febs.13286
Novel familial dilated cardiomyopathy mutation in MYL2 affects the structure and function of myosin regulatory light chain
Abstract
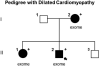
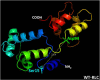
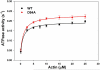
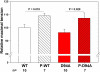
Dilated cardiomyopathy (DCM) is a disease of the myocardium characterized by left ventricular dilatation and diminished contractile function. Here we describe a novel DCM mutation in the myosin regulatory light chain (RLC), in which aspartic acid at position 94 is replaced by alanine (D94A). The mutation was identified by exome sequencing of three adult first-degree relatives who met formal criteria for idiopathic DCM. To obtain insight into the functional significance of this pathogenic MYL2 variant, we cloned and purified the human ventricular RLC wild-type (WT) and D94A mutant proteins, and performed in vitro experiments using RLC-mutant or WT-reconstituted porcine cardiac preparations. The mutation induced a reduction in the α-helical content of the RLC, and imposed intra-molecular rearrangements. The phosphorylation of RLC by Ca²⁺/calmodulin-activated myosin light chain kinase was not affected by D94A. The mutation was seen to impair binding of RLC to the myosin heavy chain, and its incorporation into RLC-depleted porcine myosin. The actin-activated ATPase activity of mutant-reconstituted porcine cardiac myosin was significantly higher compared with ATPase of wild-type. No changes in the myofibrillar ATPase-pCa relationship were observed in wild-type- or D94A-reconstituted preparations. Measurements of contractile force showed a slightly reduced maximal tension per cross-section of muscle, with no change in the calcium sensitivity of force in D94A-reconstituted skinned porcine papillary muscle strips compared with wild-type. Our data indicate that subtle structural rearrangements in the RLC molecule, followed by its impaired interaction with the myosin heavy chain, may trigger functional abnormalities contributing to the DCM phenotype.
Keywords: ATPase activity; RLC-reconstituted β-myosin; muscle contraction; phosphorylation; secondary structure.
© 2015 FEBS.
Figures













References
-
- Hershberger RE, Hedges DJ, Morales A. Dilated cardiomyopathy: the complexity of a diverse genetic architecture. Nature reviews Cardiology. 2013;10:531–47. - PubMed
-
- Millat G, Bouvagnet P, Chevalier P, Sebbag L, Dulac A, Dauphin C, Jouk PS, Delrue MA, Thambo JB, Le Metayer P, Seronde MF, Faivre L, Eicher JC, Rousson R. Clinical and mutational spectrum in a cohort of 105 unrelated patients with dilated cardiomyopathy. Eur J Med Genet. 2011;54:e570–5. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

