Role of Bacillus subtilis DNA Glycosylase MutM in Counteracting Oxidatively Induced DNA Damage and in Stationary-Phase-Associated Mutagenesis
- PMID: 25825434
- PMCID: PMC4420911
- DOI: 10.1128/JB.00147-15
Role of Bacillus subtilis DNA Glycosylase MutM in Counteracting Oxidatively Induced DNA Damage and in Stationary-Phase-Associated Mutagenesis
Abstract
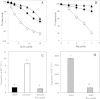
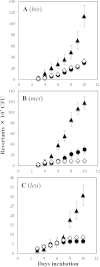
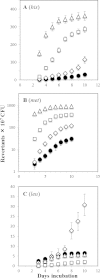
Reactive oxygen species (ROS) promote the synthesis of the DNA lesion 8-oxo-G, whose mutagenic effects are counteracted in distinct organisms by the DNA glycosylase MutM. We report here that in Bacillus subtilis, mutM is expressed during the exponential and stationary phases of growth. In agreement with this expression pattern, results of a Western blot analysis confirmed the presence of MutM in both stages of growth. In comparison with cells of a wild-type strain, cells of B. subtilis lacking MutM increased their spontaneous mutation frequency to Rif(r) and were more sensitive to the ROS promoter agents hydrogen peroxide and 1,1'-dimethyl-4,4'-bipyridinium dichloride (Paraquat). However, despite MutM's proven participation in preventing ROS-induced-DNA damage, the expression of mutM was not induced by hydrogen peroxide, mitomycin C, or NaCl, suggesting that transcription of this gene is not under the control of the RecA, PerR, or σ(B) regulons. Finally, the role of MutM in stationary-phase-associated mutagenesis (SPM) was investigated in the strain B. subtilis YB955 (hisC952 metB5 leuC427). Results revealed that under limiting growth conditions, a mutM knockout strain significantly increased the amount of stationary-phase-associated his, met, and leu revertants produced. In summary, our results support the notion that the absence of MutM promotes mutagenesis that allows nutritionally stressed B. subtilis cells to escape from growth-limiting conditions.
Importance: The present study describes the role played by a DNA repair protein (MutM) in protecting the soil bacterium Bacillus subtilis from the genotoxic effects induced by reactive oxygen species (ROS) promoter agents. Moreover, it reveals that the genetic inactivation of mutM allows nutritionally stressed bacteria to escape from growth-limiting conditions, putatively by a mechanism that involves the accumulation and error-prone processing of oxidized DNA bases.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

