A little sugar goes a long way: the cell biology of O-GlcNAc
- PMID: 25825515
- PMCID: PMC4384737
- DOI: 10.1083/jcb.201501101
A little sugar goes a long way: the cell biology of O-GlcNAc
Abstract
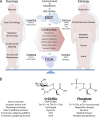
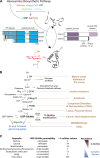
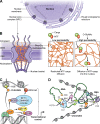
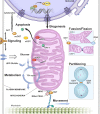
Unlike the complex glycans decorating the cell surface, the O-linked β-N-acetyl glucosamine (O-GlcNAc) modification is a simple intracellular Ser/Thr-linked monosaccharide that is important for disease-relevant signaling and enzyme regulation. O-GlcNAcylation requires uridine diphosphate-GlcNAc, a precursor responsive to nutrient status and other environmental cues. Alternative splicing of the genes encoding the O-GlcNAc cycling enzymes O-GlcNAc transferase (OGT) and O-GlcNAcase (OGA) yields isoforms targeted to discrete sites in the nucleus, cytoplasm, and mitochondria. OGT and OGA also partner with cellular effectors and act in tandem with other posttranslational modifications. The enzymes of O-GlcNAc cycling act preferentially on intrinsically disordered domains of target proteins impacting transcription, metabolism, apoptosis, organelle biogenesis, and transport.
Figures
References
-
- Bauer C., Göbel K., Nagaraj N., Colantuoni C., Wang M., Müller U., Kremmer E., Rottach A., and Leonhardt H.. 2015. Phosphorylation of TET proteins is regulated via O-GlcNAcylation by the O-linked N-acetylglucosamine transferase (OGT). J. Biol. Chem. 290:4801–4812 10.1074/jbc.M114.605881 - DOI - PMC - PubMed
-
- Boehmelt G., Fialka I., Brothers G., McGinley M.D., Patterson S.D., Mo R., Hui C.C., Chung S., Huber L.A., Mak T.W., and Iscove N.N.. 2000a. Cloning and characterization of the murine glucosamine-6-phosphate acetyltransferase EMeg32. Differential expression and intracellular membrane association. J. Biol. Chem. 275:12821–12832 10.1074/jbc.275.17.12821 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

