Obesity genetics in mouse and human: back and forth, and back again
- PMID: 25825681
- PMCID: PMC4375971
- DOI: 10.7717/peerj.856
Obesity genetics in mouse and human: back and forth, and back again
Abstract
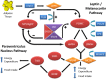
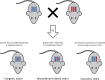
Obesity is a major public health concern. This condition results from a constant and complex interplay between predisposing genes and environmental stimuli. Current attempts to manage obesity have been moderately effective and a better understanding of the etiology of obesity is required for the development of more successful and personalized prevention and treatment options. To that effect, mouse models have been an essential tool in expanding our understanding of obesity, due to the availability of their complete genome sequence, genetically identified and defined strains, various tools for genetic manipulation and the accessibility of target tissues for obesity that are not easily attainable from humans. Our knowledge of monogenic obesity in humans greatly benefited from the mouse obesity genetics field. Genes underlying highly penetrant forms of monogenic obesity are part of the leptin-melanocortin pathway in the hypothalamus. Recently, hypothesis-generating genome-wide association studies for polygenic obesity traits in humans have led to the identification of 119 common gene variants with modest effect, most of them having an unknown function. These discoveries have led to novel animal models and have illuminated new biologic pathways. Integrated mouse-human genetic approaches have firmly established new obesity candidate genes. Innovative strategies recently developed by scientists are described in this review to accelerate the identification of causal genes and deepen our understanding of obesity etiology. An exhaustive dissection of the molecular roots of obesity may ultimately help to tackle the growing obesity epidemic worldwide.
Keywords: Genetics; Genome-wide association study; Human; Integrative biology; Knock-out; Monogenic obesity; Mouse; Next generation sequencing; Polygenic obesity; Transgenic.
Conflict of interest statement
David Meyre is an Academic Editor for PeerJ.
Figures


References
-
- Ahmad S, Rukh G, Varga TV, Ali A, Kurbasic A, Shungin D, Ericson U, Koivula RW, Chu AY, Rose LM, Ganna A, Qi Q, Stanáková A, Sandholt CH, Elks CE, Curhan G, Jensen MK, Tamimi RM, Allin KH, Jørgensen T, Brage S, Langenberg C, Aadahl M, Grarup N, Linneberg A, Paré G, Magnusson PKE, Pedersen NL, Boehnke M, Hamsten A, Mohlke KL, Pasquale LT, Pedersen O, Scott RA, Ridker PM, Ingelsson E, Laakso M, Hansen T, Qi L, Wareham NJ, Chasman DI, Hallmans G, Hu FB, Renström F, Orho-Melander M, Franks PW. Gene × physical activity interactions in obesity: combined analysis of 111,421 individuals of European ancestry. PLoS Genetics. 2013;9(7):e856. doi: 10.1371/journal.pgen.1003607. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

