SOX2 primes the epigenetic landscape in neural precursors enabling proper gene activation during hippocampal neurogenesis
- PMID: 25825708
- PMCID: PMC4403144
- DOI: 10.1073/pnas.1421480112
SOX2 primes the epigenetic landscape in neural precursors enabling proper gene activation during hippocampal neurogenesis
Abstract
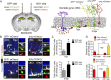
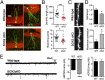
Newborn granule neurons generated from neural progenitor cells (NPCs) in the adult hippocampus play a key role in spatial learning and pattern separation. However, the molecular mechanisms that control activation of their neurogenic program remain poorly understood. Here, we report a novel function for the pluripotency factor sex-determining region Y (SRY)-related HMG box 2 (SOX2) in regulating the epigenetic landscape of poised genes activated at the onset of neuronal differentiation. We found that SOX2 binds to bivalently marked promoters of poised proneural genes [neurogenin 2 (Ngn2) and neurogenic differentiation 1 (NeuroD1)] and a subset of neurogenic genes [e.g., SRY-box 21 (Sox21), brain-derived neurotrophic factor (Bdnf), and growth arrest and DNA-damage-inducible, beta (Gadd45b)] where it functions to maintain the bivalent chromatin state by preventing excessive polycomb repressive complex 2 activity. Conditional ablation of SOX2 in adult hippocampal NPCs impaired the activation of proneural and neurogenic genes, resulting in increased neuroblast death and functionally aberrant newborn neurons. We propose that SOX2 sets a permissive epigenetic state in NPCs, thus enabling proper activation of the neuronal differentiation program under neurogenic cue.
Keywords: SOX2; epigenetics; neurogenesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

