The circadian oscillator in Synechococcus elongatus controls metabolite partitioning during diurnal growth
- PMID: 25825710
- PMCID: PMC4403147
- DOI: 10.1073/pnas.1504576112
The circadian oscillator in Synechococcus elongatus controls metabolite partitioning during diurnal growth
Abstract
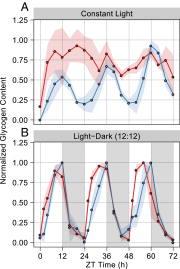
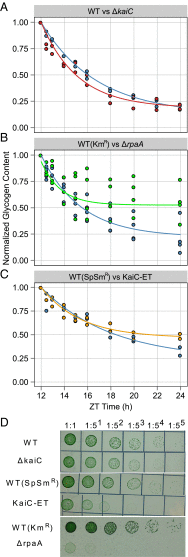
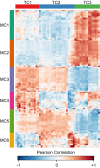
Synechococcus elongatus PCC 7942 is a genetically tractable model cyanobacterium that has been engineered to produce industrially relevant biomolecules and is the best-studied model for a prokaryotic circadian clock. However, the organism is commonly grown in continuous light in the laboratory, and data on metabolic processes under diurnal conditions are lacking. Moreover, the influence of the circadian clock on diurnal metabolism has been investigated only briefly. Here, we demonstrate that the circadian oscillator influences rhythms of metabolism during diurnal growth, even though light-dark cycles can drive metabolic rhythms independently. Moreover, the phenotype associated with loss of the core oscillator protein, KaiC, is distinct from that caused by absence of the circadian output transcriptional regulator, RpaA (regulator of phycobilisome-associated A). Although RpaA activity is important for carbon degradation at night, KaiC is dispensable for those processes. Untargeted metabolomics analysis and glycogen kinetics suggest that functional KaiC is important for metabolite partitioning in the morning. Additionally, output from the oscillator functions to inhibit RpaA activity in the morning, and kaiC-null strains expressing a mutant KaiC phosphomimetic, KaiC-pST, in which the oscillator is locked in the most active output state, phenocopies a ΔrpaA strain. Inhibition of RpaA by the oscillator in the morning suppresses metabolic processes that normally are active at night, and kaiC-null strains show indications of oxidative pentose phosphate pathway activation as well as increased abundance of primary metabolites. Inhibitory clock output may serve to allow secondary metabolite biosynthesis in the morning, and some metabolites resulting from these processes may feed back to reinforce clock timing.
Keywords: circadian clock; cyanobacteria; diurnal; metabolism; metabolomics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Redox crisis underlies conditional light-dark lethality in cyanobacterial mutants that lack the circadian regulator, RpaA.Proc Natl Acad Sci U S A. 2017 Jan 24;114(4):E580-E589. doi: 10.1073/pnas.1613078114. Epub 2017 Jan 10. Proc Natl Acad Sci U S A. 2017. PMID: 28074036 Free PMC article.
-
Active output state of the Synechococcus Kai circadian oscillator.Proc Natl Acad Sci U S A. 2013 Oct 1;110(40):E3849-57. doi: 10.1073/pnas.1315170110. Epub 2013 Sep 16. Proc Natl Acad Sci U S A. 2013. PMID: 24043774 Free PMC article.
-
Involvement of glycogen metabolism in circadian control of UV resistance in cyanobacteria.PLoS Genet. 2020 Nov 30;16(11):e1009230. doi: 10.1371/journal.pgen.1009230. eCollection 2020 Nov. PLoS Genet. 2020. PMID: 33253146 Free PMC article.
-
Diversity of KaiC-based timing systems in marine Cyanobacteria.Mar Genomics. 2014 Apr;14:3-16. doi: 10.1016/j.margen.2013.12.006. Epub 2014 Jan 3. Mar Genomics. 2014. PMID: 24388874 Review.
-
A cyanobacterial circadian clock based on the Kai oscillator.Cold Spring Harb Symp Quant Biol. 2007;72:47-55. doi: 10.1101/sqb.2007.72.029. Cold Spring Harb Symp Quant Biol. 2007. PMID: 18419262 Review.
Cited by
-
Improved sugar-free succinate production by Synechocystis sp. PCC 6803 following identification of the limiting steps in glycogen catabolism.Metab Eng Commun. 2016 May 3;3:130-141. doi: 10.1016/j.meteno.2016.04.003. eCollection 2016 Dec. Metab Eng Commun. 2016. PMID: 29468119 Free PMC article.
-
Dynamic Inventory of Intermediate Metabolites of Cyanobacteria in a Diurnal Cycle.iScience. 2020 Oct 20;23(11):101704. doi: 10.1016/j.isci.2020.101704. eCollection 2020 Nov 20. iScience. 2020. PMID: 33196027 Free PMC article.
-
Diurnal Regulation of Cellular Processes in the Cyanobacterium Synechocystis sp. Strain PCC 6803: Insights from Transcriptomic, Fluxomic, and Physiological Analyses.mBio. 2016 May 3;7(3):e00464-16. doi: 10.1128/mBio.00464-16. mBio. 2016. PMID: 27143387 Free PMC article.
-
Culturing Synechocystis sp. Strain PCC 6803 with N2 and CO2 in a Diel Regime Reveals Multiphase Glycogen Dynamics with Low Maintenance Costs.Appl Environ Microbiol. 2016 Jun 30;82(14):4180-4189. doi: 10.1128/AEM.00256-16. Print 2016 Jul 15. Appl Environ Microbiol. 2016. PMID: 27208121 Free PMC article.
-
Synchronization of the circadian clock to the environment tracked in real time.Proc Natl Acad Sci U S A. 2023 Mar 28;120(13):e2221453120. doi: 10.1073/pnas.2221453120. Epub 2023 Mar 20. Proc Natl Acad Sci U S A. 2023. PMID: 36940340 Free PMC article.
References
-
- Atsumi S, Higashide W, Liao JC. Direct photosynthetic recycling of carbon dioxide to isobutyraldehyde. Nat Biotechnol. 2009;27(12):1177–1180. - PubMed
-
- Schirmer A, Rude MA, Li X, Popova E, del Cardayre SB. Microbial biosynthesis of alkanes. Science. 2010;329(5991):559–562. - PubMed
-
- Kruse O, Hankamer B. Microalgal hydrogen production. Curr Opin Biotechnol. 2010;21(3):238–243. - PubMed
-
- Wijffels RH, Kruse O, Hellingwerf KJ. Potential of industrial biotechnology with cyanobacteria and eukaryotic microalgae. Curr Opin Biotechnol. 2013;24(3):405–413. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

