Zinc as chaperone-mimicking agent for retardation of amyloid β peptide fibril formation
- PMID: 25825723
- PMCID: PMC4418866
- DOI: 10.1073/pnas.1421961112
Zinc as chaperone-mimicking agent for retardation of amyloid β peptide fibril formation
Abstract
Metal ions have emerged to play a key role in the aggregation process of amyloid β (Aβ) peptide that is closely related to the pathogenesis of Alzheimer's disease. A detailed understanding of the underlying mechanistic process of peptide-metal interactions, however, has been challenging to obtain. By applying a combination of NMR relaxation dispersion and fluorescence kinetics methods we have investigated quantitatively the thermodynamic Aβ-Zn(2+) binding features as well as how Zn(2+) modulates the nucleation mechanism of the aggregation process. Our results show that, under near-physiological conditions, substoichiometric amounts of Zn(2+) effectively retard the generation of amyloid fibrils. A global kinetic profile analysis reveals that in the absence of zinc Aβ40 aggregation is driven by a monomer-dependent secondary nucleation process in addition to fibril-end elongation. In the presence of Zn(2+), the elongation rate is reduced, resulting in reduction of the aggregation rate, but not a complete inhibition of amyloid formation. We show that Zn(2+) transiently binds to residues in the N terminus of the monomeric peptide. A thermodynamic analysis supports a model where the N terminus is folded around the Zn(2+) ion, forming a marginally stable, short-lived folded Aβ40 species. This conformation is highly dynamic and only a few percent of the peptide molecules adopt this structure at any given time point. Our findings suggest that the folded Aβ40-Zn(2+) complex modulates the fibril ends, where elongation takes place, which efficiently retards fibril formation. In this conceptual framework we propose that zinc adopts the role of a minimal antiaggregation chaperone for Aβ40.
Keywords: Alzheimer’s disease; NMR relaxation; aggregation kinetics; amyloid beta peptide; zinc ion interactions.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
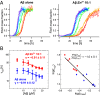
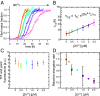
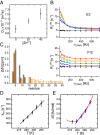

Comment in
-
Preventing peptide and protein misbehavior.Proc Natl Acad Sci U S A. 2015 Apr 28;112(17):5267-8. doi: 10.1073/pnas.1505170112. Epub 2015 Apr 20. Proc Natl Acad Sci U S A. 2015. PMID: 25902542 Free PMC article. No abstract available.
References
-
- Chiti F, Dobson CM. Protein misfolding, functional amyloid, and human disease. Annu Rev Biochem. 2006;75:333–366. - PubMed
-
- Benilova I, De Strooper B. An overlooked neurotoxic species in Alzheimer’s disease. Nat Neurosci. 2011;14(8):949–950. - PubMed
-
- Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: Lessons from the Alzheimer’s amyloid beta-peptide. Nat Rev Mol Cell Biol. 2007;8(2):101–112. - PubMed
-
- Bush AI, et al. Rapid induction of Alzheimer A beta amyloid formation by zinc. Science. 1994;265(5177):1464–1467. - PubMed
-
- Ayton S, Lei P, Bush AI. Metallostasis in Alzheimer’s disease. Free Radic Biol Med. 2013;62:76–89. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

