Tectal microcircuit generating visual selection commands on gaze-controlling neurons
- PMID: 25825743
- PMCID: PMC4403191
- DOI: 10.1073/pnas.1504866112
Tectal microcircuit generating visual selection commands on gaze-controlling neurons
Abstract
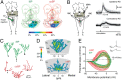
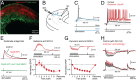
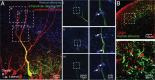
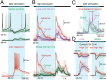
The optic tectum (called superior colliculus in mammals) is critical for eye-head gaze shifts as we navigate in the terrain and need to adapt our movements to the visual scene. The neuronal mechanisms underlying the tectal contribution to stimulus selection and gaze reorientation remains, however, unclear at the microcircuit level. To analyze this complex--yet phylogenetically conserved--sensorimotor system, we developed a novel in vitro preparation in the lamprey that maintains the eye and midbrain intact and allows for whole-cell recordings from prelabeled tectal gaze-controlling cells in the deep layer, while visual stimuli are delivered. We found that receptive field activation of these cells provide monosynaptic retinal excitation followed by local GABAergic inhibition (feedforward). The entire remaining retina, on the other hand, elicits only inhibition (surround inhibition). If two stimuli are delivered simultaneously, one inside and one outside the receptive field, the former excitatory response is suppressed. When local inhibition is pharmacologically blocked, the suppression induced by competing stimuli is canceled. We suggest that this rivalry between visual areas across the tectal map is triggered through long-range inhibitory tectal connections. Selection commands conveyed via gaze-controlling neurons in the optic tectum are, thus, formed through synaptic integration of local retinotopic excitation and global tectal inhibition. We anticipate that this mechanism not only exists in lamprey but is also conserved throughout vertebrate evolution.
Keywords: GABAergic inhibition; evolution; gaze control; optic tectum; superior colliculus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Van Opstal AJ, Van Gisbergen JAM. A nonlinear model for collicular spatial interactions underlying the metrical properties of electrically elicited saccades. Biol Cybern. 1989;60(3):171–183. - PubMed
-
- Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annu Rev Neurosci. 1995;18:193–222. - PubMed
-
- Findlay JM, Walker R. A model of saccade generation based on parallel processing and competitive inhibition. Behav Brain Sci. 1999;22(4):661–674, discussion 674–721. - PubMed
-
- Itti L, Koch C. Computational modelling of visual attention. Nat Rev Neurosci. 2001;2(3):194–203. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

