Integrative, dynamic structural biology at atomic resolution--it's about time
- PMID: 25825836
- PMCID: PMC4457290
- DOI: 10.1038/nmeth.3324
Integrative, dynamic structural biology at atomic resolution--it's about time
Abstract
Biomolecules adopt a dynamic ensemble of conformations, each with the potential to interact with binding partners or perform the chemical reactions required for a multitude of cellular functions. Recent advances in X-ray crystallography, nuclear magnetic resonance (NMR) spectroscopy and other techniques are helping us realize the dream of seeing--in atomic detail--how different parts of biomolecules shift between functional substates using concerted motions. Integrative structural biology has advanced our understanding of the formation of large macromolecular complexes and how their components interact in assemblies by leveraging data from many low-resolution methods. Here, we review the growing opportunities for integrative, dynamic structural biology at the atomic scale, contending there is increasing synergistic potential between X-ray crystallography, NMR and computer simulations to reveal a structural basis for protein conformational dynamics at high resolution.
Conflict of interest statement
Competing financial interests statement
We declare no competing financial interests.
Figures
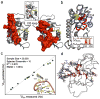
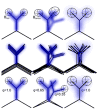



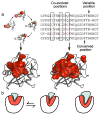

References
-
- Boehr DD, McElheny D, Dyson HJ, Wright PE. The dynamic energy landscape of dihydrofolate reductase catalysis. Science. 2006;313:1638–42. CPMG relaxation dispersion experiments characterize the structural dynamics of the catalytic cycle of DHFR as a sequence of ‘linked’ ground and excited states. - PubMed
-
- Henzler-Wildman KA, et al. Intrinsic motions along an enzymatic reaction trajectory. Nature. 2007;450:838–44. - PubMed
-
- Linder M, Johansson AJ, Olsson TSG, Liebeschuetz J, Brinck T. Computational design of a Diels-Alderase from a thermophilic esterase: the importance of dynamics. J Comput Aided Mol Des. 2012;26:1079–95. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

