Breaking the hydrophobicity of the MscL pore: insights into a charge-induced gating mechanism
- PMID: 25825909
- PMCID: PMC4380313
- DOI: 10.1371/journal.pone.0120196
Breaking the hydrophobicity of the MscL pore: insights into a charge-induced gating mechanism
Abstract
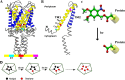
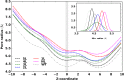
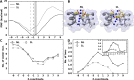
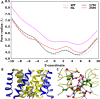
The mechanosensitive channel of large conductance (MscL) is a protein that responds to membrane tension by opening a transient pore during osmotic downshock. Due to its large pore size and functional reconstitution into lipid membranes, MscL has been proposed as a promising artificial nanovalve suitable for biotechnological applications. For example, site-specific mutations and tailored chemical modifications have shown how MscL channel gating can be triggered in the absence of tension by introducing charged residues at the hydrophobic pore level. Recently, engineered MscL proteins responsive to stimuli like pH or light have been reported. Inspired by experiments, we present a thorough computational study aiming at describing, with atomistic detail, the artificial gating mechanism and the molecular transport properties of a light-actuated bacterial MscL channel, in which a charge-induced gating mechanism has been enabled through the selective cleavage of photo-sensitive alkylating agents. Properties such as structural transitions, pore dimension, ion flux and selectivity have been carefully analyzed. Besides, the effects of charge on alternative sites of the channel with respect to those already reported have been addressed. Overall, our results provide useful molecular insights into the structural events accompanying the engineered MscL channel gating and the interplay of electrostatic effects, channel opening and permeation properties. In addition, we describe how the experimentally observed ionic current in a single-subunit charged MscL mutant is obtained through a hydrophobicity breaking mechanism involving an asymmetric inter-subunit motion.
Conflict of interest statement
Figures

References
-
- Blount P, Moe PC (1999) Bacterial mechanosensitive channels: integrating physiology, structure and function. Trends in microbiology 7: 420–424. - PubMed
-
- Perozo E, Rees DC (2003) Structure and mechanism in prokaryotic mechanosensitive channels. Current Opinion in Structural Biology 13: 432–442. - PubMed
-
- Chang G, Spencer RH, Lee AT, Barclay MT, Rees DC (1998) Structure of the MscL Homolog from Mycobacterium tuberculosis: A Gated Mechanosensitive Ion Channel. Science 282: 2220–2226. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

