Specific medicinal plant polysaccharides effectively enhance the potency of a DC-based vaccine against mouse mammary tumor metastasis
- PMID: 25825910
- PMCID: PMC4380423
- DOI: 10.1371/journal.pone.0122374
Specific medicinal plant polysaccharides effectively enhance the potency of a DC-based vaccine against mouse mammary tumor metastasis
Abstract
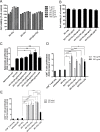
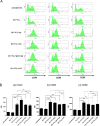
Dendritic cell (DC) vaccines are a newly emerging immunotherapeutic approach for the treatment and prevention of cancer, but major challenges still remain particularly with respect to clinical efficacy. Engineering and optimization of adjuvant formulations for DC-based vaccines is one strategy through which more efficacious treatments may be obtained. In this study, we developed a new ex vivo approach for DC vaccine preparation. We evaluated two highly purified mixed polysaccharide fractions from the root of Astragalus membranaceus and Codonopsis pilosulae, named Am and Cp, for their use in enhancing the efficiency of a DC-based cancer vaccine against metastasis of 4T1 mammary carcinoma in mice. Mixed lymphocyte reaction showed all Am-, Cp- and [Am+Cp]-treated DCs enhanced mouse CD4+ and CD8+ T-cell proliferation. [Am+Cp]-treated DCs exhibited the strongest anti-4T1 metastasis activity in test mice. Treatments with Am, Cp and [Am+Cp] also resulted in augmented expression of CD40, CD80 and CD86 markers in test DCs. Bioinformatics analysis of the cytokine array data from treated DCs identified that [Am+Cp] is efficacious in activation of specific immune functions via mediating the expression of cytokines/chemokines involved in the recruitment and differentiation of defined immune cells. Biochemical analysis revealed that Am and Cp are composed mainly of polysaccharides containing a high level (70-95%) glucose residues, but few or no (< 1%) mannose residues. In summary, our findings suggest that the specific plant polysaccharides Am and Cp extracted from traditional Chinese medicines can be effectively used instead of bacterial LPS as a potent adjuvant in the formulation of a DC-based vaccine for cancer immunotherapies.
Conflict of interest statement
Figures




Similar articles
-
Portulaca oleracea L. polysaccharides enhance the immune efficacy of dendritic cell vaccine for breast cancer.Food Funct. 2021 May 11;12(9):4046-4059. doi: 10.1039/d0fo02522d. Food Funct. 2021. PMID: 33977945
-
Rapamycin Promotes Mouse 4T1 Tumor Metastasis that Can Be Reversed by a Dendritic Cell-Based Vaccine.PLoS One. 2015 Oct 1;10(10):e0138335. doi: 10.1371/journal.pone.0138335. eCollection 2015. PLoS One. 2015. PMID: 26426423 Free PMC article.
-
Phenotypic profile of dendritic and T cells in the lymph node of Balb/C mice with breast cancer submitted to dendritic cells immunotherapy.Immunol Lett. 2016 Sep;177:25-37. doi: 10.1016/j.imlet.2016.07.009. Epub 2016 Jul 14. Immunol Lett. 2016. PMID: 27423825
-
Dendritic cell gene therapy.Surg Oncol Clin N Am. 2002 Jul;11(3):645-60. doi: 10.1016/s1055-3207(02)00027-3. Surg Oncol Clin N Am. 2002. PMID: 12487060 Review.
-
Application of dendritic cells in tumor immunotherapy and progress in the mechanism of anti-tumor effect of Astragalus polysaccharide (APS) modulating dendritic cells: a review.Biomed Pharmacother. 2022 Nov;155:113541. doi: 10.1016/j.biopha.2022.113541. Epub 2022 Sep 18. Biomed Pharmacother. 2022. PMID: 36127221 Review.
Cited by
-
Natural Products as Anticancer Agents: Current Status and Future Perspectives.Molecules. 2022 Nov 30;27(23):8367. doi: 10.3390/molecules27238367. Molecules. 2022. PMID: 36500466 Free PMC article. Review.
-
Chinese nonmedicinal herbal diet and risk of nasopharyngeal carcinoma: A population-based case-control study.Cancer. 2019 Dec 15;125(24):4462-4470. doi: 10.1002/cncr.32458. Epub 2019 Sep 22. Cancer. 2019. PMID: 31544233 Free PMC article.
-
Natural polysaccharides exert anti-tumor effects as dendritic cell immune enhancers.Front Oncol. 2023 Oct 9;13:1274048. doi: 10.3389/fonc.2023.1274048. eCollection 2023. Front Oncol. 2023. PMID: 37876967 Free PMC article. Review.
-
Impact of TCM on Tumor-Infiltrating Myeloid Precursors in the Tumor Microenvironment.Front Cell Dev Biol. 2021 Mar 4;9:635122. doi: 10.3389/fcell.2021.635122. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33748122 Free PMC article. Review.
-
Research Progress on the Anticancer Activity of Plant Polysaccharides.Recent Pat Anticancer Drug Discov. 2024;19(5):573-598. doi: 10.2174/1574892819666230915103434. Recent Pat Anticancer Drug Discov. 2024. PMID: 37724671 Review.
References
-
- Cardoso F, Harbeck N, Fallowfield L, Kyriakides S, Senkus E, ESMO Guidelines Working Group. Locally recurrent or metastatic breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23 Suppl 7: vii11–19. - PubMed
-
- Vulink A, Radford KJ, Melief C, Hart DNJ. Dendritic Cells in Cancer Immunotherapy. 2008; 99: 363–407. - PubMed
-
- Park JW, Melisko ME, Esserman LJ, Jones LA, Wollan JB, Sims R. Treatment with autologous antigen-presenting cells activated with the HER-2 based antigen Lapuleucel-T: results of a phase I study in immunologic and clinical activity in HER-2 overexpressing breast cancer. J Clin Oncol. 2007;25: 3680–3687. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

