Antibody and lectin target podoplanin to inhibit oral squamous carcinoma cell migration and viability by distinct mechanisms
- PMID: 25826087
- PMCID: PMC4496201
- DOI: 10.18632/oncotarget.3515
Antibody and lectin target podoplanin to inhibit oral squamous carcinoma cell migration and viability by distinct mechanisms
Abstract
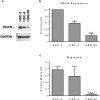
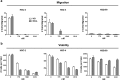
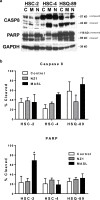
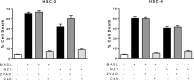
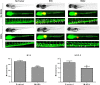
Podoplanin (PDPN) is a unique transmembrane receptor that promotes tumor cell motility. Indeed, PDPN may serve as a chemotherapeutic target for primary and metastatic cancer cells, particularly oral squamous cell carcinoma (OSCC) cells that cause most oral cancers. Here, we studied how a monoclonal antibody (NZ-1) and lectin (MASL) that target PDPN affect human OSCC cell motility and viability. Both reagents inhibited the migration of PDPN expressing OSCC cells at nanomolar concentrations before inhibiting cell viability at micromolar concentrations. In addition, both reagents induced mitochondrial membrane permeability transition to kill OSCC cells that express PDPN by caspase independent nonapoptotic necrosis. Furthermore, MASL displayed a surprisingly robust ability to target PDPN on OSCC cells within minutes of exposure, and significantly inhibited human OSCC dissemination in zebrafish embryos. Moreover, we report that human OSCC cells formed tumors that expressed PDPN in mice, and induced PDPN expression in infiltrating host murine cancer associated fibroblasts. Taken together, these data suggest that antibodies and lectins may be utilized to combat OSCC and other cancers that express PDPN.
Keywords: cancer; cell migration; lectin; podoplanin; receptor.
Figures

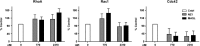



Similar articles
-
Effects of Maackia amurensis seed lectin (MASL) on OSCC cell morphology, PDPN expression, growth, and motility in a phase 1 clinical trial.J Cancer Res Clin Oncol. 2025 Jul 19;151(7):218. doi: 10.1007/s00432-025-06265-z. J Cancer Res Clin Oncol. 2025. PMID: 40681758 Free PMC article. Clinical Trial.
-
Maackia amurensis seed lectin (MASL) and soluble human podoplanin (shPDPN) sequence analysis and effects on human oral squamous cell carcinoma (OSCC) cell migration and viability.Biochem Biophys Res Commun. 2024 May 28;710:149881. doi: 10.1016/j.bbrc.2024.149881. Epub 2024 Apr 3. Biochem Biophys Res Commun. 2024. PMID: 38583233 Free PMC article.
-
Insulin-like growth factor-II mRNA binding protein-3 and podoplanin expression are associated with bone invasion and prognosis in oral squamous cell carcinoma.Arch Oral Biol. 2016 Sep;69:25-32. doi: 10.1016/j.archoralbio.2016.05.008. Epub 2016 May 12. Arch Oral Biol. 2016. PMID: 27232357
-
Platelet CLEC2-Podoplanin Axis as a Promising Target for Oral Cancer Treatment.Front Immunol. 2021 Dec 15;12:807600. doi: 10.3389/fimmu.2021.807600. eCollection 2021. Front Immunol. 2021. PMID: 34987523 Free PMC article. Review.
-
Podoplanin emerges as a functionally relevant oral cancer biomarker and therapeutic target.Oral Oncol. 2018 Mar;78:126-136. doi: 10.1016/j.oraloncology.2018.01.011. Epub 2018 Feb 20. Oral Oncol. 2018. PMID: 29496040 Review.
Cited by
-
Differences in cardiovascular toxicities associated with cigarette smoking and snuff use revealed using novel zebrafish models.Biol Open. 2016 Jul 15;5(7):970-8. doi: 10.1242/bio.018812. Biol Open. 2016. PMID: 27334697 Free PMC article.
-
New Therapeutic Strategies for Osteoarthritis by Targeting Sialic Acid Receptors.Biomolecules. 2020 Apr 21;10(4):637. doi: 10.3390/biom10040637. Biomolecules. 2020. PMID: 32326143 Free PMC article.
-
Single cell time-lapse analysis reveals that podoplanin enhances cell survival and colony formation capacity of squamous cell carcinoma cells.Sci Rep. 2017 Jan 6;7:39971. doi: 10.1038/srep39971. Sci Rep. 2017. PMID: 28059107 Free PMC article.
-
Maackia amurensis seed lectin (MASL) ameliorates articular cartilage destruction and increases movement velocity of mice with TNFα induced rheumatoid arthritis.Biochem Biophys Rep. 2022 Sep 9;32:101341. doi: 10.1016/j.bbrep.2022.101341. eCollection 2022 Dec. Biochem Biophys Rep. 2022. PMID: 36120492 Free PMC article.
-
Podoplanin Expression in Canine Melanoma.Monoclon Antib Immunodiagn Immunother. 2016 Dec;35(6):304-306. doi: 10.1089/mab.2016.0040. Epub 2016 Dec 5. Monoclon Antib Immunodiagn Immunother. 2016. PMID: 27918691 Free PMC article.
References
-
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer JClin. 2011;61(2):69–90. - PubMed
-
- Johnson NW, Jayasekara P, Amarasinghe AA. Squamous cell carcinoma and precursor lesions of the oral cavity: epidemiology and aetiology. Periodontol2000. 2011;57(1):19–37. - PubMed
-
- Warnakulasuriya S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 2009;45(4-5):309–316. - PubMed
-
- Inoue H, Miyazaki Y, Kikuchi K, Yoshida N, Ide F, Ohmori Y, Tomomura A, Sakashita H, Kusama K. Podoplanin expression during dysplasia-carcinoma sequence in the oral cavity. TumourBiol. 2012;33(1):183–194. - PubMed
-
- Kreppel M, Drebber U, Wedemeyer I, Eich HT, Backhaus T, Zoller JE, Scheer M. Podoplanin expression predicts prognosis in patients with oral squamous cell carcinoma treated with neoadjuvant radiochemotherapy. Oral Oncol. 2011;47(9):873–878. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

