Electrical stimulation alleviates depressive-like behaviors of rats: investigation of brain targets and potential mechanisms
- PMID: 25826110
- PMCID: PMC4354354
- DOI: 10.1038/tp.2015.24
Electrical stimulation alleviates depressive-like behaviors of rats: investigation of brain targets and potential mechanisms
Abstract
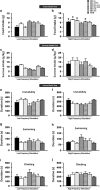
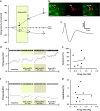
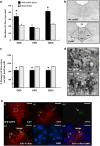
Deep brain stimulation (DBS) is a promising therapy for patients with refractory depression. However, key questions remain with regard to which brain target(s) should be used for stimulation, and which mechanisms underlie the therapeutic effects. Here, we investigated the effect of DBS, with low- and high-frequency stimulation (LFS, HFS), in different brain regions (ventromedial prefrontal cortex, vmPFC; cingulate cortex, Cg; nucleus accumbens (NAc) core or shell; lateral habenula, LHb; and ventral tegmental area) on a variety of depressive-like behaviors using rat models. In the naive animal study, we found that HFS of the Cg, vmPFC, NAc core and LHb reduced anxiety levels and increased motivation for food. In the chronic unpredictable stress model, there was a robust depressive-like behavioral phenotype. Moreover, vmPFC HFS, in a comparison of all stimulated targets, produced the most profound antidepressant effects with enhanced hedonia, reduced anxiety and decreased forced-swim immobility. In the following set of electrophysiological and histochemical experiments designed to unravel some of the underlying mechanisms, we found that vmPFC HFS evoked a specific modulation of the serotonergic neurons in the dorsal raphe nucleus (DRN), which have long been linked to mood. Finally, using a neuronal mapping approach by means of c-Fos expression, we found that vmPFC HFS modulated a brain circuit linked to the DRN and known to be involved in affect. In conclusion, HFS of the vmPFC produced the most potent antidepressant effects in naive rats and rats subjected to stress by mechanisms also including the DRN.
Figures

 ) indicates the tips of all electrode localization. cc, corpus callosum; Cg1 and Cg2, cingulate cortex 1 and cingulate cortex 2; D3V, dorsal third ventricle; fmi, forceps minor of corpus callosum; fr, fasciculus retroflexus; IF, interfascicular nucleus; IL, infralimbic; LHb, lateral habenular nucleus; MHb, medial habenular nucleus; M2, secondary motor cortex; NAc core, nucleus accumbens core; NAc shell, nucleus accumbens shell; PrL, prelimbic; RLi, rostral linear nucleus of the raphe; RPC, parvicellular part of the red nucleus; SNR, reticular part of the substantia nigra; vmPFC, ventromedial prefrontal cortex; VTA, ventral tegmental area.
) indicates the tips of all electrode localization. cc, corpus callosum; Cg1 and Cg2, cingulate cortex 1 and cingulate cortex 2; D3V, dorsal third ventricle; fmi, forceps minor of corpus callosum; fr, fasciculus retroflexus; IF, interfascicular nucleus; IL, infralimbic; LHb, lateral habenular nucleus; MHb, medial habenular nucleus; M2, secondary motor cortex; NAc core, nucleus accumbens core; NAc shell, nucleus accumbens shell; PrL, prelimbic; RLi, rostral linear nucleus of the raphe; RPC, parvicellular part of the red nucleus; SNR, reticular part of the substantia nigra; vmPFC, ventromedial prefrontal cortex; VTA, ventral tegmental area.

References
-
- Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) JAMA. 2003;289:3095–3105. - PubMed
-
- Alonso J, Angermeyer MC, Bernert S, Bruffaerts R, Brugha TS, Bryson H, et al. Prevalence of mental disorders in Europe: results from the European Study of the Epidemiology of Mental Disorders (ESEMeD) project. Acta Psychiatr Scand Suppl. 2004;420:21–27. - PubMed
-
- American Psychiatric Association Practice guideline for the treatment of patients with major depressive disorder (revision) Am J Psychiatry. 2002;157:1–45. - PubMed
-
- Temel Y, Hescham SA, Jahanshahi A, Janssen ML, Tan SK, van Overbeeke JJ, et al. Neuromodulation in psychiatric disorders. Int Rev Neurobiol. 2012;107:283–314. - PubMed
-
- Temel Y, Lim LW. Neurosurgical treatments of depression. Curr Top Behav Neurosci. 2013;14:327–339. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

