Development and validation of a risk score for chronic kidney disease in HIV infection using prospective cohort data from the D:A:D study
- PMID: 25826420
- PMCID: PMC4380415
- DOI: 10.1371/journal.pmed.1001809
Development and validation of a risk score for chronic kidney disease in HIV infection using prospective cohort data from the D:A:D study
Abstract
Background: Chronic kidney disease (CKD) is a major health issue for HIV-positive individuals, associated with increased morbidity and mortality. Development and implementation of a risk score model for CKD would allow comparison of the risks and benefits of adding potentially nephrotoxic antiretrovirals to a treatment regimen and would identify those at greatest risk of CKD. The aims of this study were to develop a simple, externally validated, and widely applicable long-term risk score model for CKD in HIV-positive individuals that can guide decision making in clinical practice.
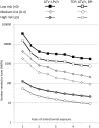
Methods and findings: A total of 17,954 HIV-positive individuals from the Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D) study with ≥3 estimated glomerular filtration rate (eGFR) values after 1 January 2004 were included. Baseline was defined as the first eGFR > 60 ml/min/1.73 m2 after 1 January 2004; individuals with exposure to tenofovir, atazanavir, atazanavir/ritonavir, lopinavir/ritonavir, other boosted protease inhibitors before baseline were excluded. CKD was defined as confirmed (>3 mo apart) eGFR ≤ 60 ml/min/1.73 m2. Poisson regression was used to develop a risk score, externally validated on two independent cohorts. In the D:A:D study, 641 individuals developed CKD during 103,185 person-years of follow-up (PYFU; incidence 6.2/1,000 PYFU, 95% CI 5.7-6.7; median follow-up 6.1 y, range 0.3-9.1 y). Older age, intravenous drug use, hepatitis C coinfection, lower baseline eGFR, female gender, lower CD4 count nadir, hypertension, diabetes, and cardiovascular disease (CVD) predicted CKD. The adjusted incidence rate ratios of these nine categorical variables were scaled and summed to create the risk score. The median risk score at baseline was -2 (interquartile range -4 to 2). There was a 1:393 chance of developing CKD in the next 5 y in the low risk group (risk score < 0, 33 events), rising to 1:47 and 1:6 in the medium (risk score 0-4, 103 events) and high risk groups (risk score ≥ 5, 505 events), respectively. Number needed to harm (NNTH) at 5 y when starting unboosted atazanavir or lopinavir/ritonavir among those with a low risk score was 1,702 (95% CI 1,166-3,367); NNTH was 202 (95% CI 159-278) and 21 (95% CI 19-23), respectively, for those with a medium and high risk score. NNTH was 739 (95% CI 506-1462), 88 (95% CI 69-121), and 9 (95% CI 8-10) for those with a low, medium, and high risk score, respectively, starting tenofovir, atazanavir/ritonavir, or another boosted protease inhibitor. The Royal Free Hospital Clinic Cohort included 2,548 individuals, of whom 94 individuals developed CKD (3.7%) during 18,376 PYFU (median follow-up 7.4 y, range 0.3-12.7 y). Of 2,013 individuals included from the SMART/ESPRIT control arms, 32 individuals developed CKD (1.6%) during 8,452 PYFU (median follow-up 4.1 y, range 0.6-8.1 y). External validation showed that the risk score predicted well in these cohorts. Limitations of this study included limited data on race and no information on proteinuria.
Conclusions: Both traditional and HIV-related risk factors were predictive of CKD. These factors were used to develop a risk score for CKD in HIV infection, externally validated, that has direct clinical relevance for patients and clinicians to weigh the benefits of certain antiretrovirals against the risk of CKD and to identify those at greatest risk of CKD.
Conflict of interest statement
PM: honoraria or travel/meeting expenses from Bristol-Myers Squibb, Gilead, Janssen-Cilag, Merck Sharp & Dohme-Chibret and ViiV Healthcare in the past five years. ML received unrestricted grants to my institution from Boehringer Ingelhiem, Gilead Sciences, Merck Sharp & Dohme, Bristol-Myers Squibb, Janssen-Cilag, ViiV HealthCare. CS has received funds for preparation of Educational Materials from Janssen, Gilead, BMS, ViiV HealthCare, support for attendance at Ad board and support to attend HIV conference from Gilead, funding to conduct study from BMS. OM has received honoraria as a speaker from Abbott and Gilead Sciences, serves on the board of Roche, and had expenses paid by Roche and Baxter for travel, accommodations, and meetings. OK has received honoraria, consultancy, lecture fees, and travel grants from Abbott Laboratories, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck Sharp & Dohme, Roche, and ViiV Healthcare, and has served/is serving on Advisory Boards for Gilead Sciences, Merck Sharp & Dohme, and ViiV Healthcare. AM has received honoraria, consultancy, lecture fees, and travel grants from Gilead Sciences, Merck Sharp & Dohme, Boehringer Ingelheim, Pfizer and GSK.
Figures

Comment in
-
Risk factors: Individual assessment of CKD risk in HIV-positive patients.Nat Rev Nephrol. 2015 Jul;11(7):392-3. doi: 10.1038/nrneph.2015.75. Epub 2015 May 12. Nat Rev Nephrol. 2015. PMID: 25963593 No abstract available.
References
-
- Mocroft A, Vella S, Benfield TL, Chiesi A, Miller V, et al. (1998) Changing patterns of mortality across Europe in patients infected with HIV-1. EuroSIDA Study Group. Lancet 352: 1725–1730. - PubMed
-
- McAlister FA, Ezekowitz J, Tonelli M, Armstrong PW (2004) Renal insufficiency and heart failure: prognostic and therapeutic implications from a prospective cohort study. Circulation 109: 1004–1009. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U01 AI068641/AI/NIAID NIH HHS/United States
- 5U01AI046362-03/AI/NIAID NIH HHS/United States
- U01-AI46957/AI/NIAID NIH HHS/United States
- U01 AI046957/AI/NIAID NIH HHS/United States
- 5U01AI042170-10/AI/NIAID NIH HHS/United States
- UM1-AI068641/AI/NIAID NIH HHS/United States
- UM1 AI068641/AI/NIAID NIH HHS/United States
- U01 AI042170/AI/NIAID NIH HHS/United States
- P30 AI094189/AI/NIAID NIH HHS/United States
- U01-AI042170/AI/NIAID NIH HHS/United States
- U01-AI068641/AI/NIAID NIH HHS/United States
- U01-AI069907/AI/NIAID NIH HHS/United States
- U01 AI046362/AI/NIAID NIH HHS/United States
- U01 AI069907/AI/NIAID NIH HHS/United States
- U01-AI46362/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

