Irisin, a Novel Myokine, Regulates Glucose Uptake in Skeletal Muscle Cells via AMPK
- PMID: 25826445
- PMCID: PMC5414737
- DOI: 10.1210/me.2014-1353
Irisin, a Novel Myokine, Regulates Glucose Uptake in Skeletal Muscle Cells via AMPK
Abstract
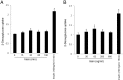
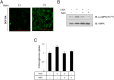
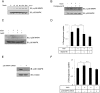
Irisin is a novel myokine produced by skeletal muscle. However, its metabolic role is poorly understood. In the present study, irisin induced glucose uptake in differentiated skeletal muscle cells. It increased AMP-activated protein kinase (AMPK) phosphorylation and the inhibition of AMPK blocked glucose uptake. It also increased reactive oxygen species (ROS) generation. N-acetyl cysteine, a ROS scavenger, blocked irisin-induced AMPK phosphorylation. Moreover, irisin activated p38 MAPK in an AMPK-dependent manner. The inhibition and knockdown of p38 MAPK blocked irisin-induced glucose uptake. A colorimetric absorbance assay showed that irisin stimulated the translocation of glucose transporter type 4 to the plasma membrane and that this effect was suppressed in cells pretreated with a p38 MAPK inhibitor or p38 MAPK small interfering RNA. In primary cultured myoblast cells, irisin increased the concentration of intracellular calcium. STO-609, a calcium/calmodulin-dependent protein kinase kinase inhibitor, blocked irisin-induced AMPK phosphorylation, implying that calcium is involved in irisin-mediated signaling. Our results suggest that irisin plays an important role in glucose metabolism via the ROS-mediated AMPK pathway in skeletal muscle cells.
Figures



References
-
- Hardie DG, Carling D. The AMP-activated protein kinase–fuel gauge of the mammalian cell? Eur J Biochem. 1997;246:259–273. - PubMed
-
- Woods A, Dickerson K, Heath R, et al. . Ca2+/calmodulin-dependent protein kinase kinase-β acts upstream of AMP-activated protein kinase in mammalian cells. Cell Metab. 2005;2:21–33. - PubMed
-
- Hawley SA, Pan DA, Mustard KJ, et al. . Calmodulin-dependent protein kinase kinase-β is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab. 2005;2:9–19. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

