Buprenorphine for treating cancer pain
- PMID: 25826743
- PMCID: PMC6513197
- DOI: 10.1002/14651858.CD009596.pub4
Buprenorphine for treating cancer pain
Abstract
Background: Many patients with cancer experience moderate to severe pain that requires treatment with strong analgesics. Buprenorphine, fentanyl and morphine are examples of strong opioids used for cancer pain relief. However, strong opioids are ineffective as pain treatment in all patients and are not well-tolerated by all patients. The aim of this Cochrane review is to assess whether buprenorphine is associated with superior, inferior or equal pain relief and tolerability compared to other analgesic options for patients with cancer pain.
Objectives: To assess the effectiveness and tolerability of buprenorphine for pain in adults and children with cancer.
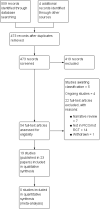
Search methods: We searched CENTRAL (the Cochrane Library) issue 12 or 12 2014, MEDLINE (via OVID) 1948 to 20 January 2015, EMBASE (via OVID) 1980 to 20 January 2015, ISI Web of Science (SCI-EXPANDED & CPCI-S) to 20 January 2015, ISI BIOSIS 1969 to 20 January 2015. We also searched ClinicalTrials.gov (http://clinicaltrials.gov/; metaRegister of Controlled Trials (mRCT) (http://www.controlled-trials.com/mrct/), the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) search portal (http://apps.who.int/trialsearch/) and the Proceedings of the Congress of the European Federation of International Association for the Study of Pain (IASP; via European Journal of Pain Supplements) on 16 February 2015. We checked the bibliographic references of identified studies as well as relevant studies and systematic reviews to find additional trials not identified by the electronic searches. We contacted authors of included studies for other relevant studies.
Selection criteria: We included randomised controlled trials, with parallel-group or crossover design, comparing buprenorphine (any formulation and any route of administration) with placebo or an active drug (including buprenorphine) for cancer background pain in adults and children.
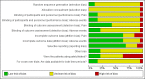
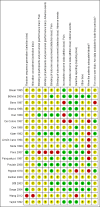
Data collection and analysis: Two review authors independently extracted data pertaining to study design, participant details (including age, cancer characteristics, previous analgesic medication and setting), interventions (including details about titration) and outcomes, and independently assessed the quality of the included studies according to standard Cochrane methodology. As it was not feasible to meta-analyse the data, we summarised the results narratively. We assessed the overall quality of the evidence for each outcome using the GRADE approach.
Main results: In this Cochrane review we identified 19 relevant studies including a total of 1421 patients that examined 16 different intervention comparisons.Of the studies that compared buprenorphine to another drug, 11 studies performed comparative analyses between the randomised groups, and five studies found that buprenorphine was superior to the comparison treatment. Three studies found no differences between buprenorphine and the comparison drug, while another three studies found treatment with buprenorphine to be inferior to the alternative treatment in terms of the side effects profile or patients preference/acceptability.Of the studies that compared different doses or formulations/routes of administration of buprenorphine, pain intensity ratings did not differ significantly between intramuscular buprenorphine and buprenorphine suppository. However, the average severity of dizziness, nausea, vomiting and adverse events as a total were all significantly higher in the intramuscular group relatively to the suppository group (one study).Sublingual buprenorphine was associated with faster onset of pain relief compared to subdermal buprenorphine, with similar duration analgesia and no significant differences in adverse event rates reported between the treatments (one study).In terms of transdermal buprenorphine, two studies found it superior to placebo, whereas a third study found no difference between placebo and different doses of transdermal buprenorphine.The studies that examined different doses of transdermal buprenorphine did not report a clear dose-response relationship.The quality of this evidence base was limited by under-reporting of most bias assessment items (e.g., the patient selection items), by small sample sizes in several included studies, by attrition (with data missing from 8.2% of the enrolled/randomised patients for efficacy and from 14.6% for safety) and by limited or no reporting of the expected outcomes in a number of cases. The evidence for all the outcomes was very low quality.
Authors' conclusions: Based on the available evidence, it is difficult to say where buprenorphine fits in the treatment of cancer pain with strong opioids. However, it might be considered to rank as a fourth-line option compared to the more standard therapies of morphine, oxycodone and fentanyl, and even there it would only be suitable for some patients. However, palliative care patients are often heterogeneous and complex, so having a number of analgesics available that can be given differently increases patient and prescriber choice. In particular, the sublingual and injectable routes seemed to have a more definable analgesic effect, whereas the transdermal route studies left more questions.
Conflict of interest statement
Mia Schmidt‐Hansen has no conflicts of interest to declare. Nathan Bromham has no conflicts of interest to declare. Mark Taubert has no conflicts of interest to declare. Stephanie Arnold has no conflicts of interest to declare. Jennifer S Hilgart has no conflicts of interest to declare.
Figures
Update of
References
References to studies included in this review
Bauer 1985 {published data only}
-
- Bauer M, Schmid H, Schulz‐Wentland R. Gynecologic carcinoma patients with chronic pain. Comparison of sublingual buprenorphine with tilidine plus naloxone. Therapiewoche 1985;35(35):3943‐7.
Böhme 2003 {published data only}
-
- Boehme K. Buprenorphine transdermal system (TDS) (Delivery rates 35 / 52.5 70 mg/h) in comparison to sublingual buprenorphine in chronic pain patients. Journal of Pain and Symptom Management 2000;20:S54.
-
- Böhme K, Likar R. Efficacy and tolerability of a new opioid analgesic formulation, buprenorphine transdermal therapeutic system (TDS), in the treatment of patients with chronic pain. A randomised, double‐blind, placebo‐controlled study. The Pain Clinic 2003;15(2):193‐202.
Bono 1997 {published data only}
-
- Bono AV, Cuffari S. [Effectiveness and tolerance of tramadol in cancer pain. A comparative study with respect to buprenorphine]. Drugs 1997;53(Suppl 2):40‐9. - PubMed
Brema 1996 {published data only}
-
- Brema F, Pastorino G, Martini MC, Gottlieb A, Luzzani M, Libretti A, et al. Oral tramadol and buprenorphine in tumour pain. An Italian multicentre trial. International Journal of Clinical Pharmacology Research 1996;16(4‐5):109‐16. - PubMed
Dan 1989 {published data only}
-
- Dan K, Yoshitake J, Tashiro H, Kurihara Y, Yoshida T, Fukushima K, et al. Double blind comparative study on 0.2mg suppository and 0.2mg injection preparation of buprenorphine hydrochloride for light to middle degree cancer pain. Igaku no Ayumi 1989;148(6):435‐46.
De Conno 1987 {published data only}
-
- Conno F, Ripamonti C, Tamburini M, Ventafridda V. Buprenorphine in the treatment of cancer pain: Comparison with pentazocine [Buprenorfina nel dolore da cancro: confronto incrociato con la pentazocina]. Minerva Medica 1987;78(15):1177‐81. - PubMed
-
- Ventafridda V, Conno F, Guarise G, Tamburini M, Savio G. Chronic analgesic study on buprenorphine action in cancer pain. Comparison with pentazocine. Arzneimittel‐Forschung 1983;33(4):587‐90. - PubMed
Dini 1986 {published data only}
-
- Dini D, Fassio T, Gottlieb A, Gini M. Controlled study of the analgesic effect and tolerability of buprenorphine in cancer patients [Italian]. Minerva Medica 1986;77(3‐4):93‐104. - PubMed
Kjaer 1982 {published data only}
-
- Kjaer M, Henriksen H, Knudsen EJ. Intramuscular buprenorphine and morphine in the treatment of cancer pain. A controlled study [Danish]. Ugeskrift for Laeger 1982;144(18):1306‐9. - PubMed
Limón Cano 1994 {published data only}
-
- Limón Cano S, Martínez Gómez JL, Vicencio Serrano RE, García Cuevas M, Carreón García J. Subdermic and sublingual buprenorphine for the control of cancer pain [Buprenorfina sublingual y subdermica para el control del dolor por cancer]. Revista Mexicana de Anestesiología 1994;17(4):170‐2.
Noda 1989 {published data only}
-
- Noda J, Umeda S, Arai T, Harima A, Mori K. Continuous subcutaneous infusion of buprenorphine for cancer pain control. Clinical Journal of Pain 1989;5(2):147‐52. - PubMed
Pace 2007 {published data only}
-
- Pace MC, Passavanti MB, Grella E, Mazzariello L, Maisto M, Barbarisi M, et al. Buprenorphine in long‐term control of chronic pain in cancer patients. Frontiers in Bioscience 2007;12:1291‐9. - PubMed
Pasqualucci 1987 {published data only}
-
- Pasqualucci V, Tantucci C, Paoletti F, Dottorini ML, Bifarini G, Belfiori R, et al. Buprenorphine vs. morphine via the epidural route: a controlled comparative clinical study of respiratory effects and analgesic activity. Pain 1987;29(3):273‐86. - PubMed
Poulain 2008 {published data only}
-
- Poulain P, Denier W, Douma J, Hoerauf K, Samija M, Sopata M, et al. Efficacy and safety of transdermal buprenorphine: a randomized, placebo‐controlled trial in 289 patients with severe cancer pain. Journal of Pain and Symptom Management 2008;36(2):117‐25. - PubMed
Rigolot 1979 {published data only}
-
- Rigolot JC, Vergnes Y, Bouket JL, Gauthier‐Lafaye P. [Comparative study about the analgesic effect of buprenorphine (author's transl)]. Anesthésie, Analgésie, Réanimation 1979;36(7‐8):317‐21. - PubMed
Sarhan 2009 {published data only}
-
- Sarhan T, Doghem M. A comparison of two trans‐dermal drug delivery systems; Buprenorphine and fentanyl for chronic cancer pain management. European Journal of Pain 2009;13:S199.
Sittl 2003 {published data only}
-
- Sittl R, Griessinger N, Likar R. Analgesic efficacy and tolerability of transdermal buprenorphine in patients with inadequately controlled chronic pain related to cancer and other disorders: a multicenter, randomized, double‐blind, placebo‐controlled trial. Clinical Therapeutics 2003;25(1):150‐68. - PubMed
Sorge 2004 {published data only}
-
- Sorge J. Buprenorphine transdermal system (TDS) (delivery rate 35 mg/h) in comparison to sublingual tablets in chronic pain patients. Journal of Pain and Symptom Management 2000;20:S78.
-
- Sorge J, Sittl R. Transdermal buprenorphine in the treatment of chronic pain: results of a phase III, multicenter, randomized, double‐blind, placebo‐controlled study. Clinical Therapeutics 2004;26(11):1808‐20. - PubMed
Wang 2012 {published data only}
-
- Wang J, Cai B, Huang DX, Yang SD, Guo L. Decreased analgesic effect of morphine, but not buprenorphine, in patients with advanced P‐glycoprotein(+) cancers. Pharmacological Reports: PR 2012;64(4):870‐7. - PubMed
Yajnik 1992 {published data only}
-
- Yajnik S, Singh GP, Singh G, Kumar M. Phenytoin as a coanalgesic in cancer pain. Journal of Pain and Symptom Management 1992;7(4):209‐13. - PubMed
References to studies excluded from this review
Corli 1988 {published data only}
-
- Corli O, Roma G, Battaiotto L, Bernoni M. Buprenorphine and diclofenac in cancer pain relief. Schmerz/Pain/Douleur 1988;9:114‐5.
De Conno 1993 {published data only}
-
- Conno F, Ripamonti C, Sbanotto A, Barletta L. A clinical note on sublingual buprenorphine. Journal of Palliative Care 1993;9:44‐46. - PubMed
De Conno 1997 {published data only}
-
- Conno F. Analgesic efficacy and side effects of opioides in different routes of administration for terminal cancer patients: A clinical, pharmacokinetic and neurophysiological study. New Trends in Experimental and Clinical Psychiatry 1997;13:49‐51.
Deng 2002 {published data only}
-
- Deng YP, Xu GZ, Wang W, Shen XH, Chen QT, Gao HZ, et al. Clinical evaluation of analgesic effect and safety of scorpion venom injection [Chinese]. Chinese Pharmaceutical Journal 2002;37(6):459‐62.
Enig 1983 {published data only}
-
- Enig B. Sublingual buprenorphine in the treatment of pain caused by cancer. Transition to buprenorphine from other opiates. Ugeskrift for Laeger 1983;145:3236‐3240. - PubMed
Evans 2003 {published data only}
-
- Evans HC, Easthope SE. Transdermal buprenorphine. Drugs 2003;63:1999‐2010. - PubMed
Gantsev 1994 {published data only}
-
- Gantsev SK, Vaisman MA, Gazizov AA. Prolonged sacral anesthesia with buprenorphine in combination with a local anesthetic in the treatment of chronic pain of oncologic patients with lesions of organs of the small pelvis. Anesteziologiia i Reanimatologiia, Anesteziologiia i Reanimatologiia 1994;4:50‐51. - PubMed
Hans 2007 {published data only}
-
- Hans G. Buprenorphine ‐ A review of its role in neuropathic pain. Journal of Opioid Management 2007;3:195‐206. - PubMed
Hans 2009 {published data only}
Hayek 2011 {published data only}
-
- Hayek SM, Deer TR, Pope JE, Panchal SJ, Patel V. Intrathecal therapy for cancer and non‐cancer pain. Pain Physician 2011;14:219‐248. - PubMed
Likar 2006 {published data only}
-
- Likar R, Kayser H, Sittl R. Long‐term management of chronic pain with transdermal buprenorphine: a multicenter, open‐label, follow‐up study in patients from three short‐term clinical trials. Clinical Therapeutics 2006;28(6):943‐52. - PubMed
Likar 2007 {published data only}
-
- Likar R, Lorenz V, Korak‐Leiter M, Kager I, Sittl R. Transdermal buprenorphine patches applied in a 4‐day regimen versus a 3‐day regimen: a single‐site, Phase III, randomized, open‐label, crossover comparison. Clinical Therapeutics 2007;29(8):1591‐606. - PubMed
Michel 2011 {published data only}
-
- Michel E, Anderson BJ, Zernikow B. Buprenorphine TTS for children ‐ A review of the drug's clinical pharmacology. Paediatric Anaesthesia 2011;21:280‐290. - PubMed
Radbruch 2003 {published data only}
-
- Radbruch L, Vielvoye‐Kerkmeer A. Buprenorphine TDS: The clinical development ‐ Rationale and results. International Journal of Clinical Practice 2003;Supplement:15‐18. - PubMed
Ritzman 2008 {published data only}
-
- Ritzmann P. Retarded highly effective opioid analgesics [Retardierte hochwirksame opioid‐analgetika]. Pharma‐Kritik 2008;30:61‐62.
Robbie 1979 {published data only}
Taguchi 1982 {published data only}
-
- Taguchi T. Effect of a long‐acting analgesic, buprenorphine on cancer pain‐‐a single‐blind crossover comparison with pentazocine [Japanese]. Gan To Kagaku Ryoho (Japanese Journal of Cancer & Chemotherapy) 1982;9(2):250‐7. - PubMed
Van Dongen 1999 {published data only}
-
- Dongen RTM, Crul BJP, Egmond J. Intrathecal coadministration of bupivacaine diminishes morphine dose progression during long‐term intrathecal infusion in cancer patients. Clinical Journal of Pain 1999;15:166‐172. - PubMed
Wallenstein 1986 {published data only}
-
- Wallenstein SL, Kaiko RF, Rogers AG, Houde RW. Crossover trials in clinical analgesic assays: studies of buprenorphine and morphine. Pharmacotherapy:The Journal of Human Pharmacology & Drug Therapy 1986;6:228‐235. - PubMed
Wirz 2009 {published data only}
-
- Wirz S, Wittmann M, Schenk M, Schroeck A, Schaefer N, Mueller M, et al. Gastrointestinal symptoms under opioid therapy: a prospective comparison of oral sustained‐release hydromorphone, trasndermal fentanyl, and transdermal buprenorphine. European Journal of Pain 2009;13(7):737‐43. - PubMed
Yarlas 2013 {published data only}
-
- Yarlas A, Miller K, Wen W, Kowalski M, Lynch S, Dain B, et al. Long‐term maintenance of improvements in patient‐reported functioning, quality‐of‐life, and pain outcomes for moderate‐to‐severe chronic pain patients receiving continuous treatment of Butrans (buprenorphine) Transdermal System (BTDS). Journal of Pain 2013;14:S80.
Zeppetella 2007 {published data only}
-
- Zeppetella G Wiffen PJ. Buprenorphine for cancer pain. Cochrane Database of Systematic Reviews 2007;withdrawn.
References to studies awaiting assessment
Lari 1985 {published data only}
-
- Lari S, Fabbri G, Mattioli R, Elmar K. Epidural buprenorphine versus morphine in bone cancer pain [Italian]. Minerva Anestesiologica 1985;51(11‐12):609‐14. - PubMed
Ripamonti 1987 {published data only}
-
- Ripamonti C, Conno F, Tamburini M, Ventafridda V. Acute study of parenteral buprenorphine versus parenteral morphine [Italian]. Minerva Anestesiologica 1987;53(4):135‐9. - PubMed
Staquet 1990 {published data only}
-
- Staquet M, Wasch G. A double‐blind comparison of multiple dose regiment of ketorolac and buprenorphine in patients with cancer pain. Pain 1990;Suppl 5:s353.
Wallenstein 1982 {published data only}
-
- Wallenstein SL, Kaiko RF, Rogers AG, Houde RW. Clinical analgesic assays of buprenorphine and morphine. Clinical Pharmacology and Therapeutics 1982;31(2):278.
References to ongoing studies
2008‐002273‐12 {published data only}
-
- Long term opioid administration in oncologic chronic pain: open label, prospective study on efficacy, safety and pharmacogenetic factors.. Ongoing study Not reported..
2008‐003592‐48 {published data only}
-
- A double‐blind, multi‐centre, reference‐controlled, randomised Phase III study to compare the analgesic efficacy and tolerability of a buprenorphine transdermal system in two different application intervals using three different dosages (35, 52.5 or 70 µg/h) in patients with chronic, severe cancer pain inadequately controlled with other analgesics.. Ongoing study 25 November 2008..
NCT00916890 {published data only}
-
- Chronic Administration of Opioids in Cancer Chronic Pain:an Open Prospective Study on Efficacy, Safety and Pharmacogenetic Factors Influence.. Ongoing study February 2009..
NCT01809106 {published data only}
-
- RCT Comparing the Analgesic Efficacy of 4 Therapeutic Strategies Based on 4 Different Major Opioids (Fentanyl, Oxycodone, Buprenorphine vs Morphine) in Cancer Patients With Moderate/Severe Pain, at the Moment of Starting 3rd Step of WHO Analgesic Ladder.. Ongoing study April 2011..
Additional references
Bach 1991
-
- Bach V, Kamp‐Jensen M, Jensen NH, Eriksen J. Buprenorphine and sustained release morphine ‐ Effect and side‐effects in chronic use. Pain Clinic 1991;4:87‐93.
Bennett 2008
-
- Bennett MI. What evidence do we have that the WHO analgesic ladder is effective in cancer pain?. In: McQuay HJ, Moore R, Kalso E editor(s). Systematic Reviews in Pain Research; Methodology Refined. Seattle: IASP Press, 2008:303‐13.
Budd 2004
-
- Budd K, Shipton E. Acute pain and the immune system and opioimmunosuppression. Acute Pain 2004;6:123‐35.
Bullingham 1981
Bullingham 1983
-
- Bullingham RES, McQuay HJ, Moore RA. Clinical pharmacokinetics of narcotic agonist‐antagonist drugs. Clinical Pharmacology 1983;8(4):332‐43. - PubMed
Dahan 2005
-
- Dahan A, Yassen A, Bijl H, Romberg R, Sarton E, Teppema L, et al. Comparison of the respiratory effects of intravenous buprenorphine and fentanyl in humans and rats. British Journal of Anaesthesia 2005;94(6):825‐34. - PubMed
Dahan 2006
-
- Dahan A, Yassen A, Romberg R, Sarton E, Teppema L, Olofsen E, et al. Buprenorphine induces ceiling in respiratory depression but not in analgesia. British Journal of Anaesthetics 2006;96(5):627‐32. - PubMed
Dahan 2010
-
- Dahan A, Aarts L, Smith TW. Incidence, reversal and prevention of opioid‐induced respiratory depression. Anesthesiology 2010;112(1):226‐38. - PubMed
Deandra 2008
Deandrea 2009
Elkader 2005
-
- Elkader A, Sproule B. Buprenorphine: clinical pharmacokinetics in the treatment of opioid dependence. Clinical Pharmacokinetics 2005;44(7):661‐80. - PubMed
Ferlay 2010
-
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. International Journal of Cancer 2010;127(12):2893‐917. - PubMed
Filitz 2006
-
- Filitz J, Griessinger N, Sittl R, Likar R, Schüttler J, Koppert W. Effect of intermittent hemodialysis on buprenorphine and norbuprenorphine plasma concentrations in chronic pain patients treated with transdermal buprenorphine. European Journal of Pain 2006;10(8):743‐8. - PubMed
Foley 1998
-
- Foley KM. Pain assessment and cancer pain syndromes. In: Doyle D, Hanks GWC, MacDonald N editor(s). Oxford Textbook of Palliative Medicine. Oxford: Oxford University Press, 1998:310‐31.
Foster 2013
-
- Foster B, Twycross R, Mihalyo M, Wilcock A. Buprenorphine. Journal of Pain and Symptom Management 2013;45(5):939‐49. - PubMed
Frankish 2003
-
- Frankish H. 15 million new cancer cases per year by 2020, says WHO. Lancet 2003;361(9365):1278. - PubMed
Gal 1989
-
- Gal TJ. Naloxone reversal of buprenorphine‐induced respiratory depression. Clinical Pharmacology and Therapeutics 1989;45(1):66‐71. - PubMed
Guyatt 2008
Hadley 2013
Hand 1990
-
- Hand CW, Sear JW, Uppington J, Ball MJ, McQuay HJ, Moore RA. Buprenorphine disposition in patients with renal impairment: single and continuous dosing, with special reference to metabolites. British Journal of Anaesthesia 1990;64(3):276‐82. - PubMed
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Lewis 2004
-
- Lewis JW, Husbands SM. The orvinols and related opioids‐high affinity ligands with diverse efficacy profiles. Current Pharmaceutical Design 2004;10(7):717‐32. - PubMed
Likar 2008
-
- Likar R, Krainer B, Sittl R. Challenging the equipotency calculation for transdermal buprenorphine: four case studies. International Journal of Clinical Practice 2008;62(1):152‐6. - PubMed
Mercadante 2009
-
- Mercadante S, Casuccio A, Tirelli W, Giarratano A. Equipotent doses to switch from high doses of opioids to transdermal buprenorphine. Support Care Cancer 2009;17(6):715‐8. - PubMed
Moore 2013
-
- Moore RA, Straube S, Aldington D. Pain measures and cut‐offs ‐ 'no worse than mild pain' as a simple, universal outcome. Anaesthesia 2013;68(4):400‐12. - PubMed
NICE 2012
-
- National Institute for Health and Care Excellence. Opioids in palliative care. Clinical guidelines CG140. Issued: May 2012. www.nice.org.uk/cg140 (accessed 7 November 2013).
Nicholson 2007
Pergolizzi 2008
-
- Pergolizzi J, Böger RH, Budd K, Dahan A, Erdine S, Hans G, et al. Opioids and the management of chronic severe pain in the elderly: consensus statement of an International Expert Panel with focus on the six clinically most often used World Health Organization Step III opioids (buprenorphine, fentanyl, hydromorphone, methadone, morphine, oxycodone). Pain Practice 2008;8(4):287‐313. - PubMed
Portenoy 1989
-
- Portenoy RK. Cancer pain. Epidemiology and syndromes. Cancer 1989;63(11 Suppl):2298‐307. - PubMed
Quigley 2002
Review Manager 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Robbie 1979
Rothman 1995
-
- Rothman R. A review of the binding literature. In: Cowan A, Lewis JW editor(s). Buprenorphine Combating Drug Abuse with a Unique Opioid. New York: Wiley‐Liss, 1995:19‐29.
Sacerdote 2000
-
- Sacerdote P, Bianchi M, Gaspani L, Manfredi B, Maucione A, Terno G, et al. The effects of tramadol and morphine on immune responses and pain after surgery in cancer patients. Anesthesia & Analgesia 2000;90(6):1411‐4. - PubMed
Sacerdote 2008
-
- Sacerdote P, Franchi S, Gerra G, Leccese V, Panerai AE, Somaini L. Buprenorphine and methadone maintenance treatment of heroin addicts preserves immune function. Brain, Behavior, and Immunity 2008;22(4):606‐13. - PubMed
Schmidt‐Hansen 2015
Seya 2011
-
- Seya MJ, Gelders SF, Achara OU, Milani B, Scholten WK. A first comparison between the consumption of and the need for opioid analgesics at country, regional, and global levels. Journal of Pain and Palliative Care Pharmacotherapy 2011;25(1):6‐18. - PubMed
Sittl 2005
-
- Sittl R, Likar R, Nautrup BP. Equipotent doses of transdermal fentanyl and transdermal buprenorphine in patients with cancer and noncancer pain: results of a retrospective cohort study. Clinical Therapeutics 2005;27(2):225‐37. - PubMed
Staritz 1986
Tassinari 2008
-
- Tassinari D, Sartori S, Tamburini E, Scarpi E, Raffaeli W, Tombesi P, et al. Adverse effects of transdermal opiates treating moderate‐severe cancer pain in comparison to long‐acting morphine: a meta‐analysis and systematic review of the literature. Journal of Palliative Medicine 2008;11(3):492‐501. - PubMed
Tassinari 2011
-
- Tassinari D, Drudi F, Rosati M, Maltoni M. Transdermal opioids as front line treatment of moderate to severe cancer pain: a systemic review. Palliative Medicine 2011;25(5):478‐87. - PubMed
van den Beuken‐van Everdingen 2007
-
- Beuken‐van Everdingen MH, Rijke JM, Kessels AG, Schouten HC, Kleef M, Patijn J. Prevalence of pain in patients with cancer: a systematic review of the past 40 years. Annals of Oncology 2007;18(9):1437‐49. - PubMed
Ventafridda 1987
-
- Ventafridda V, Tamburini M, Caraceni A, Conno F, Naldi F. A validation study of the WHO method for cancer pain relief. Cancer 1987;59(4):851‐6. - PubMed
WHO 1987
-
- World Health Organization. Traitement de la douleur cancéreuse. Geneva, Switzerland: World Health Organization, 1987.
WHO 1996
-
- World Health Organization. Cancer pain relief. 1996. http://whqlibdoc.who.int/publications/9241544821.pdf (accessed 07 November 2013).
Wiffen 2013
Wolff 2012
-
- Wolff RF, Reid K, Nisio M, Aune D, Truyers C, Hernandez AV, et al. Systematic review of adverse events of buprenorphine patch versus fentanyl patch in patients with chronic moderate‐to‐severe pain. Pain Management 2012;2(4):351‐62. - PubMed
World Bank 2013
-
- World Bank. How we classify countries. http://data.worldbank.org/about/country‐classifications (accessed 07 November 2013).
Zaki 2000
-
- Zaki P, Keith DE Jr, Brine GA, Carroll FI, Evans CJ. Ligand‐induced changes in surface mu‐opioid receptor number: relationship to G protein activation?. Journal of Pharmacology and Experimental Therapeutics 2000;292(3):1127‐34. - PubMed
References to other published versions of this review
Naing 2012
-
- Naing C‐M, Aung K, Yeoh PN. Buprenorphine for treating cancer pain. Cochrane Database of Systematic Reviews 2012, Issue 1. [DOI: 10.1002/14651858.CD009596.pub3] - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials