Brief Report: Oxidative Stress Mediates Cardiomyocyte Apoptosis in a Human Model of Danon Disease and Heart Failure
- PMID: 25826782
- PMCID: PMC4651661
- DOI: 10.1002/stem.2015
Brief Report: Oxidative Stress Mediates Cardiomyocyte Apoptosis in a Human Model of Danon Disease and Heart Failure
Abstract
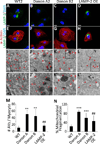
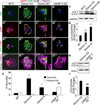
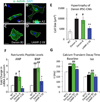
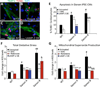
Danon disease is a familial cardiomyopathy associated with impaired autophagy due to mutations in the gene encoding lysosomal-associated membrane protein type 2 (LAMP-2). Emerging evidence has highlighted the importance of autophagy in regulating cardiomyocyte bioenergetics, function, and survival. However, the mechanisms responsible for cellular dysfunction and death in cardiomyocytes with impaired autophagic flux remain unclear. To investigate the molecular mechanisms responsible for Danon disease, we created induced pluripotent stem cells (iPSCs) from two patients with different LAMP-2 mutations. Danon iPSC-derived cardiomyocytes (iPSC-CMs) exhibited impaired autophagic flux and key features of heart failure such as increased cell size, increased expression of natriuretic peptides, and abnormal calcium handling compared to control iPSC-CMs. Additionally, Danon iPSC-CMs demonstrated excessive amounts of mitochondrial oxidative stress and apoptosis. Using the sulfhydryl antioxidant N-acetylcysteine to scavenge free radicals resulted in a significant reduction in apoptotic cell death in Danon iPSC-CMs. In summary, we have modeled Danon disease using human iPSC-CMs from patients with mutations in LAMP-2, allowing us to gain mechanistic insight into the pathogenesis of this disease. We demonstrate that LAMP-2 deficiency leads to an impairment in autophagic flux, which results in excessive oxidative stress, and subsequent cardiomyocyte apoptosis. Scavenging excessive free radicals with antioxidants may be beneficial for patients with Danon disease. In vivo studies will be necessary to validate this new treatment strategy.
Keywords: Apoptosis; Autophagy; Danon disease; Induced pluripotent stem cells; Oxidative stress.
© 2015 AlphaMed Press.
Conflict of interest statement
The authors indicate no potential conflicts of interest.
Figures
References
-
- Choi AM, Ryter SW, Levine B. Autophagy in human health and disease. N Engl J Med. 2013;368:651–662. - PubMed
Publication types
MeSH terms
Grants and funding
- DP2OD007464/OD/NIH HHS/United States
- R01 HL123626/HL/NHLBI NIH HHS/United States
- TR305687/TR/NCATS NIH HHS/United States
- F32 HL116068/HL/NHLBI NIH HHS/United States
- U01 HL107442/HL/NHLBI NIH HHS/United States
- R01 HL068854/HL/NHLBI NIH HHS/United States
- R01 HL68854/HL/NHLBI NIH HHS/United States
- K23 HL107755/HL/NHLBI NIH HHS/United States
- T32 HL007444/HL/NHLBI NIH HHS/United States
- 7K23HL107755/HL/NHLBI NIH HHS/United States
- U01HL107442/HL/NHLBI NIH HHS/United States
- T32 GM007198/GM/NIGMS NIH HHS/United States
- DP2 OD007464/OD/NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

