Whole-genome sequencing is more powerful than whole-exome sequencing for detecting exome variants
- PMID: 25827230
- PMCID: PMC4418901
- DOI: 10.1073/pnas.1418631112
Whole-genome sequencing is more powerful than whole-exome sequencing for detecting exome variants
Abstract
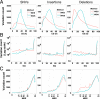
We compared whole-exome sequencing (WES) and whole-genome sequencing (WGS) in six unrelated individuals. In the regions targeted by WES capture (81.5% of the consensus coding genome), the mean numbers of single-nucleotide variants (SNVs) and small insertions/deletions (indels) detected per sample were 84,192 and 13,325, respectively, for WES, and 84,968 and 12,702, respectively, for WGS. For both SNVs and indels, the distributions of coverage depth, genotype quality, and minor read ratio were more uniform for WGS than for WES. After filtering, a mean of 74,398 (95.3%) high-quality (HQ) SNVs and 9,033 (70.6%) HQ indels were called by both platforms. A mean of 105 coding HQ SNVs and 32 indels was identified exclusively by WES whereas 692 HQ SNVs and 105 indels were identified exclusively by WGS. We Sanger-sequenced a random selection of these exclusive variants. For SNVs, the proportion of false-positive variants was higher for WES (78%) than for WGS (17%). The estimated mean number of real coding SNVs (656 variants, ∼3% of all coding HQ SNVs) identified by WGS and missed by WES was greater than the number of SNVs identified by WES and missed by WGS (26 variants). For indels, the proportions of false-positive variants were similar for WES (44%) and WGS (46%). Finally, WES was not reliable for the detection of copy-number variations, almost all of which extended beyond the targeted regions. Although currently more expensive, WGS is more powerful than WES for detecting potential disease-causing mutations within WES regions, particularly those due to SNVs.
Keywords: Mendelian disorders; exome; genetic variants; genome; next-generation sequencing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Bamshad MJ, et al. Exome sequencing as a tool for Mendelian disease gene discovery. Nat Rev Genet. 2011;12(11):745–755. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

