A comprehensive classification and nomenclature of carboxyl-carboxyl(ate) supramolecular motifs and related catemers: implications for biomolecular systems
- PMID: 25827369
- PMCID: PMC4383392
- DOI: 10.1107/S205252061500270X
A comprehensive classification and nomenclature of carboxyl-carboxyl(ate) supramolecular motifs and related catemers: implications for biomolecular systems
Abstract
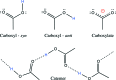
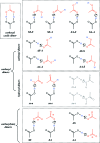
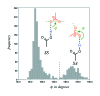
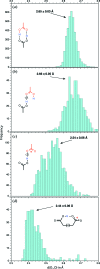
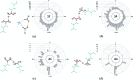
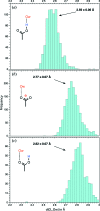
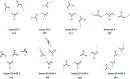
Carboxyl and carboxylate groups form important supramolecular motifs (synthons). Besides carboxyl cyclic dimers, carboxyl and carboxylate groups can associate through a single hydrogen bond. Carboxylic groups can further form polymeric-like catemer chains within crystals. To date, no exhaustive classification of these motifs has been established. In this work, 17 association types were identified (13 carboxyl-carboxyl and 4 carboxyl-carboxylate motifs) by taking into account the syn and anti carboxyl conformers, as well as the syn and anti lone pairs of the O atoms. From these data, a simple rule was derived stating that only eight distinct catemer motifs involving repetitive combinations of syn and anti carboxyl groups can be formed. Examples extracted from the Cambridge Structural Database (CSD) for all identified dimers and catemers are presented, as well as statistical data related to their occurrence and conformational preferences. The inter-carboxyl(ate) and carboxyl(ate)-water hydrogen-bond properties are described, stressing the occurrence of very short (strong) hydrogen bonds. The precise characterization and classification of these supramolecular motifs should be of interest in crystal engineering, pharmaceutical and also biomolecular sciences, where similar motifs occur in the form of pairs of Asp/Glu amino acids or motifs involving ligands bearing carboxyl(ate) groups. Hence, we present data emphasizing how the analysis of hydrogen-containing small molecules of high resolution can help understand structural aspects of larger and more complex biomolecular systems of lower resolution.
Keywords: biomolecular systems; crystal engineering; pharmaceuticals; supramolecular motifs.
Figures



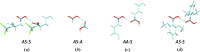

Similar articles
-
Solid state NMR studies of hydrogen bonding in a citrate synthase inhibitor complex.Biochemistry. 1999 Jun 22;38(25):8022-31. doi: 10.1021/bi9813680. Biochemistry. 1999. PMID: 10387046
-
Packing modes in some mono- and disubstituted phenylpropiolic acids: repeated occurrence of the rare syn,anti catemer.Chem Asian J. 2006 Jul 17;1(1-2):231-44. doi: 10.1002/asia.200600046. Chem Asian J. 2006. PMID: 17441060
-
Syn vs Anti Carboxylic Acids in Hybrid Peptides: Experimental and Theoretical Charge Density and Chemical Bonding Analysis.J Phys Chem A. 2018 Apr 12;122(14):3665-3679. doi: 10.1021/acs.jpca.7b10939. Epub 2018 Mar 29. J Phys Chem A. 2018. PMID: 29543470
-
Crystalline Peroxosolvates: Nature of the Coformer, Hydrogen-Bonded Networks and Clusters, Intermolecular Interactions.Molecules. 2020 Dec 23;26(1):26. doi: 10.3390/molecules26010026. Molecules. 2020. PMID: 33374602 Free PMC article. Review.
-
Coupling of electron transfer to proton uptake at the Q(B) site of the bacterial reaction center: a perspective from FTIR difference spectroscopy.Biochim Biophys Acta. 2008 Oct;1777(10):1229-48. doi: 10.1016/j.bbabio.2008.06.012. Epub 2008 Jul 11. Biochim Biophys Acta. 2008. PMID: 18671937 Review.
Cited by
-
Assessing the Conformational Equilibrium of Carboxylic Acid via Quantum Mechanical and Molecular Dynamics Studies on Acetic Acid.J Chem Inf Model. 2019 May 28;59(5):1957-1964. doi: 10.1021/acs.jcim.8b00835. Epub 2019 Feb 21. J Chem Inf Model. 2019. PMID: 30742770 Free PMC article.
-
Computational Design of Photosensitive Polymer Templates To Drive Molecular Nanofabrication.ACS Nano. 2024 Apr 9;18(14):9969-9979. doi: 10.1021/acsnano.3c10575. Epub 2024 Mar 28. ACS Nano. 2024. PMID: 38545921 Free PMC article.
-
Elucidation of the molecular interactions that enable stable assembly and structural diversity in multicomponent immune receptors.Proc Natl Acad Sci U S A. 2021 Jun 29;118(26):e2026318118. doi: 10.1073/pnas.2026318118. Proc Natl Acad Sci U S A. 2021. PMID: 34155106 Free PMC article.
-
Crystal Structure of an Archaeal Tyrosyl-tRNA Synthetase Bound to Photocaged L-Tyrosine and Its Potential Application to Time-Resolved X-ray Crystallography.Int J Mol Sci. 2022 Sep 8;23(18):10399. doi: 10.3390/ijms231810399. Int J Mol Sci. 2022. PMID: 36142308 Free PMC article.
-
Peptide Side-COOH Groups Have Two Distinct Conformations under Biorelevant Conditions.J Phys Chem Lett. 2020 May 7;11(9):3466-3472. doi: 10.1021/acs.jpclett.0c00711. Epub 2020 Apr 21. J Phys Chem Lett. 2020. PMID: 32293901 Free PMC article.
References
-
- Ahmed, H. U., Blakeley, M. P., Cianci, M., Cruickshank, D. W. J., Hubbard, J. A. & Helliwell, J. R. (2007). Acta Cryst. D63, 906–922. - PubMed
-
- Allen, F. H. (2002). Acta Cryst. B58, 380–388. - PubMed
-
- Allen, F. H. & Bruno, I. J. (2010). Acta Cryst. B66, 380–386. - PubMed
-
- Allen, F. H., Groom, C. R., Liebeschuetz, J. W., Bardwell, D. A., Olsson, T. S. & Wood, P. A. (2012). J. Chem. Inf. Model. 52, 857–866. - PubMed
-
- Allen, F. H. & Kirby, A. J. (1991). J. Am. Chem. Soc. 113, 8829–8831.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

