An educational overview of the chemistry, biochemistry and therapeutic aspects of Mn porphyrins--From superoxide dismutation to H2O2-driven pathways
- PMID: 25827425
- PMCID: PMC4392060
- DOI: 10.1016/j.redox.2015.01.017
An educational overview of the chemistry, biochemistry and therapeutic aspects of Mn porphyrins--From superoxide dismutation to H2O2-driven pathways
Erratum in
- Redox Biol. 2015 Dec;6:656
Abstract
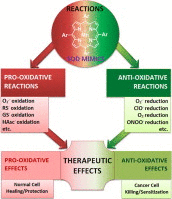
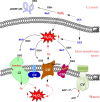
Most of the SOD mimics thus far developed belong to the classes of Mn-(MnPs) and Fe porphyrins(FePs), Mn(III) salens, Mn(II) cyclic polyamines and metal salts. Due to their remarkable stability we have predominantly explored Mn porphyrins, aiming initially at mimicking kinetics and thermodynamics of the catalysis of O2(-) dismutation by SOD enzymes. Several MnPs are of potency similar to SOD enzymes. The in vivo bioavailability and toxicity of MnPs have been addressed also. Numerous in vitro and in vivo studies indicate their impressive therapeutic efficacy. Increasing insight into complex cellular redox biology has been accompanied by increasing awareness of complex redox chemistry of MnPs. During O2(-) dismutation process, the most powerful Mn porphyrin-based SOD mimics reduce and oxidize O2(-) with close to identical rate constants. MnPs reduce and oxidize other reactive species also (none of them specific to MnPs), acting as reductants (antioxidant) and pro-oxidants. Distinction must be made between the type of reactions of MnPs and the favorable therapeutic effects we observe; the latter may be of either anti- or pro-oxidative nature. H2O2/MnP mediated oxidation of protein thiols and its impact on cellular transcription seems to dominate redox biology of MnPs. It has been thus far demonstrated that the ability of MnPs to catalyze O2(-) dismutation parallels all other reactivities (such as ONOO(-) reduction) and in turn their therapeutic efficacies. Assuming that all diseases have in common the perturbation of cellular redox environment, developing SOD mimics still seems to be the appropriate strategy for the design of potent redox-active therapeutics.
Keywords: Fe porphyrin-based SOD mimics; Mechanism(s) of action(s) of Mn porphyrins; Mn-porphyrin-based SOD mimics; Mn-porphyrin-based non-SOD mimics; Reactivities of Mn porphyrins in aqueous solutions, cells and animals; Therapeutic effects.
Copyright © 2015 The Authors. Published by Elsevier B.V. All rights reserved.
Figures





















References
-
- Batinic-Haberle I., Tovmasyan A., Roberts E.R., Vujaskovic Z., Leong K.W., Spasojevic I. SOD therapeutics: latest insights into their structure–activity relationships and impact on the cellular redox-based signaling pathways. Antioxidants and Redox Signaling. 2014;20(15):2372–2415. 23875805 - PMC - PubMed
-
- Celic T., Španjol J., Bobinac M., Tovmasyan A., Vukelic I., Reboucas J.S., Batinic-Haberle I., Bobinac D. Mn porphyrin-based SOD mimic, MnTnHex-2-PyP(5+), and non-SOD mimic, MnTBAP(3-), suppressed rat spinal cord ischemia/reperfusion injury via NF-κB pathways. Free Radical Research. 2014;48:1426–1442. 25185063 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

