Inflammatory features of pancreatic cancer highlighted by monocytes/macrophages and CD4+ T cells with clinical impact
- PMID: 25827621
- PMCID: PMC4471781
- DOI: 10.1111/cas.12663
Inflammatory features of pancreatic cancer highlighted by monocytes/macrophages and CD4+ T cells with clinical impact
Abstract
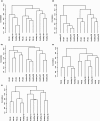
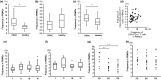
Pancreatic ductal adenocarcinoma (PDAC) is among the most fatal of malignancies with an extremely poor prognosis. The objectives of this study were to provide a detailed understanding of PDAC pathophysiology in view of the host immune response. We examined the PDAC tissues, sera, and peripheral blood cells of PDAC patients using immunohistochemical staining, the measurement of cytokine/chemokine concentrations, gene expression analysis, and flow cytometry. The PDAC tissues were infiltrated by macrophages, especially CD33+CD163+ M2 macrophages and CD4+ T cells that concomitantly express programmed cell death-1 (PD-1). Concentrations of interleukin (IL)-6, IL-7, IL-15, monocyte chemotactic protein-1, and interferon-γ-inducible protein-1 in the sera of PDAC patients were significantly elevated. The gene expression profile of CD14+ monocytes and CD4+ T cells was discernible between PDAC patients and healthy volunteers, and the differentially expressed genes were related to activated inflammation. Intriguingly, PD-1 was significantly upregulated in the peripheral blood CD4+ T cells of PDAC patients. Correspondingly, the frequency of CD4+PD-1+ T cells was increased in the peripheral blood cells of PDAC patients, and this increase correlated to chemotherapy resistance. In conclusion, inflammatory conditions in both PDAC tissue and peripheral blood cells in PDAC patients were prominent, highlighting monocytes/macrophages as well as CD4+ T cells with influence of the clinical prognosis.
Keywords: CD4+ T cells; macrophages; monocytes; pancreatic ductal adenocarcinoma; programmed cell death-1.
© 2015 The Authors. Cancer Science published by Wiley Publishing Asia Pty Ltd on behalf of Japanese Cancer Association.
Figures



References
-
- Matsuda T, Ajiki W, Marugame T, et al. Population-based survival of cancer patients diagnosed between 1993 and 1999 in Japan: a chronological and international comparative study. Jpn J Clin Oncol. 2011;41:40–51. - PubMed
-
- Paulson AS, Tran Cao HS, Tempero MA, Lowy AM. Therapeutic advances in pancreatic cancer. Gastroenterology. 2013;144:1316–26. - PubMed
-
- Reni M, Cordio S, Milandri C, et al. Gemcitabine versus cisplatin, epirubicin, fluorouracil, and gemcitabine in advanced pancreatic cancer: a randomised controlled multicentre phase III trial. Lancet Oncol. 2005;6:369–76. - PubMed
-
- Fridman WH, Pagès F, Sautès-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012;12:298–306. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

