Evidence that a neutrophil-keratinocyte crosstalk is an early target of IL-17A inhibition in psoriasis
- PMID: 25828362
- PMCID: PMC4676308
- DOI: 10.1111/exd.12710
Evidence that a neutrophil-keratinocyte crosstalk is an early target of IL-17A inhibition in psoriasis
Abstract
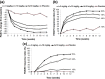
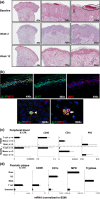
The response of psoriasis to antibodies targeting the interleukin (IL)-23/IL-17A pathway suggests a prominent role of T-helper type-17 (Th17) cells in this disease. We examined the clinical and immunological response patterns of 100 subjects with moderate-to-severe psoriasis receiving 3 different intravenous dosing regimens of the anti-IL-17A antibody secukinumab (1 × 3 mg/kg or 1 × 10 mg/kg on Day 1, or 3 × 10 mg/kg on Days 1, 15 and 29) or placebo in a phase 2 trial. Baseline biopsies revealed typical features of active psoriasis, including epidermal accumulation of neutrophils and formation of microabscesses in >60% of cases. Neutrophils were the numerically largest fraction of infiltrating cells containing IL-17 and may store the cytokine preformed, as IL-17A mRNA was not detectable in neutrophils isolated from active plaques. Significant clinical responses to secukinumab were observed 2 weeks after a single infusion, associated with extensive clearance of cutaneous neutrophils parallel to the normalization of keratinocyte abnormalities and reduction of IL-17-inducible neutrophil chemoattractants (e.g. CXCL1, CXCL8); effects on numbers of T cells and CD11c-positive dendritic cells were more delayed. Histological and immunological improvements were generally dose dependent and not observed in the placebo group. In the lowest-dose group, a recurrence of neutrophils was seen in some subjects at Week 12; these subjects relapsed faster than those without microabscesses. Our findings are indicative of a neutrophil-keratinocyte axis in psoriasis that may involve neutrophil-derived IL-17 and is an early target of IL-17A-directed therapies such as secukinumab.
Keywords: IL-17A; neutrophils; psoriasis; secukinumab.
© 2015 The Authors. Experimental Dermatology Published by John Wiley & Sons Ltd.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

