Astrocyte roles in traumatic brain injury
- PMID: 25828533
- PMCID: PMC4586307
- DOI: 10.1016/j.expneurol.2015.03.020
Astrocyte roles in traumatic brain injury
Abstract
Astrocytes sense changes in neural activity and extracellular space composition. In response, they exert homeostatic mechanisms critical for maintaining neural circuit function, such as buffering neurotransmitters, modulating extracellular osmolarity and calibrating neurovascular coupling. In addition to upholding normal brain activities, astrocytes respond to diverse forms of brain injury with heterogeneous and progressive changes of gene expression, morphology, proliferative capacity and function that are collectively referred to as reactive astrogliosis. Traumatic brain injury (TBI) sets in motion complex events in which noxious mechanical forces cause tissue damage and disrupt central nervous system (CNS) homeostasis, which in turn trigger diverse multi-cellular responses that evolve over time and can lead either to neural repair or secondary cellular injury. In response to TBI, astrocytes in different cellular microenvironments tune their reactivity to varying degrees of axonal injury, vascular disruption, ischemia and inflammation. Here we review different forms of TBI-induced astrocyte reactivity and the functional consequences of these responses for TBI pathobiology. Evidence regarding astrocyte contribution to post-traumatic tissue repair and synaptic remodeling is examined, and the potential for targeting specific aspects of astrogliosis to ameliorate TBI sequelae is considered.
Keywords: Astrocyte; Astrogliosis; CNS; Inflammation; Neuroplasticity; Scar; Traumatic brain injury.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures

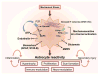
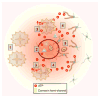

References
-
- Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7:41–53. - PubMed
-
- Ahmed SM, Rzigalinski BA, Willoughby KA, Sitterding HA, Ellis EF. Stretch-induced injury alters mitochondrial membrane potential and cellular ATP in cultured astrocytes and neurons. J Neurochem. 2000;74:1951–1960. - PubMed
-
- Akassoglou K, Probert L, Kontogeorgos G, Kollias G. Astrocyte-specific but not neuron-specific transmembrane TNF triggers inflammation and degeneration in the central nervous system of transgenic mice. J Immunol. 1997;158:438–445. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

